Allergy Asthma Immunol Res.
2017 Jan;9(1):96-98. 10.4168/aair.2017.9.1.96.
A Case of Type 2 Hereditary Angioedema With SERPING1 Mutation
- Affiliations
-
- 1Division of Allergy and Immunology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. parkjw@yuhs.ac
- KMID: 2355900
- DOI: http://doi.org/10.4168/aair.2017.9.1.96
Abstract
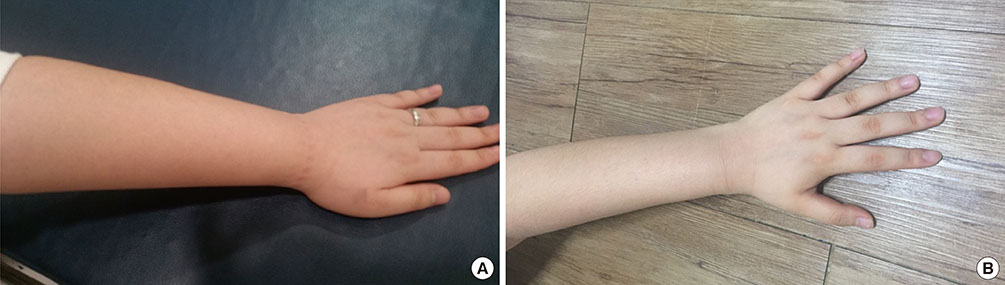
- Hereditary angioedema is a disease of congenital deficiency or functional defect in the C1 esterase inhibitor (C1-INH) consequent to mutation in the SERPING1 gene, which encodes C1-INH. This disease manifests as recurrent, non-pitting, non-pruritic subcutaneous, or submucosal edema as well as an erythematous rash in some cases. These symptoms result from the uncontrolled localized production of bradykinin. The most commonly affected sites are the extremities, face, gastrointestinal tract, and respiratory system. When the respiratory system is affected by hereditary angioedema, swelling of the airway can restrict breathing and lead to life-threatening obstruction. Herein, we report a case of a 24-year-old woman with type 2 hereditary angioedema who presented with recurrent episodic abdominal pain and swelling of the extremities. She had no family history of angioedema. Although her C4 level was markedly decreased (3.40 mg/dL; normal range: 10-40 mg/dL), she presented with a very high C1-INH level (81.0 mg/dL; normal range: 21.0-39.0 mg/dL) and abnormally low C1-INH activity (less than 25%; normal range: 70%-130%). The SERPING1 gene mutation was confirmed in this patient. She was treated with prophylactic tranexamic acid, as needed, and subsequently reported fewer and less severe episodes. To our knowledge, this is the first reported case of type 2 hereditary angioedema in Korea that was consequent to SERPING1 mutation and involved a significantly elevated level of C1-INH as well as a low level of C1-INH activity.
MeSH Terms
Figure
Reference
-
1. Agostoni A, Aygören-Pürsün E, Binkley KE, Blanch A, Bork K, Bouillet L, et al. Hereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol. 2004; 114:S51–S131.2. Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med. 2008; 359:1027–1036.3. Shibuya M, Takahashi N, Yabe M, Iwamoto K, Hide M. Hereditary angioedema as the cause of death from asphyxia: postmortem computed tomography study. Allergol Int. 2014; 63:493–494.4. Bowen T, Cicardi M, Farkas H, Bork K, Kreuz W, Zingale L, et al. Canadian 2003 international consensus algorithm for the diagnosis, therapy, and management of hereditary angioedema. J Allergy Clin Immunol. 2004; 114:629–637.5. Cichon S, Martin L, Hennies HC, Müller F, Van Driessche K, Karpushova A, et al. Increased activity of coagulation factor XII (Hageman factor) causes hereditary angioedema type III. Am J Hum Genet. 2006; 79:1098–1104.6. Lee YS, Chung JH, Cho KH, Youn JI. A case of hereditary angioedema. Korean J Dermatol. 1994; 32:115–118.7. Kim SH, Lee BJ, Chang YS, Kim YK, Cho SH, Min KU, et al. A case of hereditary angioedema associated with idiopathic hypoparathyroidism. Korean J Intern Med. 2001; 16:281–283.8. Shin M, Ahn K. A case of hereditary angioedema in a 7-year-old Korean girl. Allergy Asthma Immunol Res. 2013; 5:59–61.9. Gower RG, Busse PJ, Aygören-Pürsün E, Barakat AJ, Caballero T, Davis-Lorton M, et al. Hereditary angioedema caused by c1-esterase inhibitor deficiency: a literature-based analysis and clinical commentary on prophylaxis treatment strategies. World Allergy Organ J. 2011; 4:S9–S21.10. Davis AE 3rd. Biological effects of C1 inhibitor. Drug News Perspect. 2004; 17:439–446.11. Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G. Nonallergic angioedema: role of bradykinin. Allergy. 2007; 62:842–856.12. Fine LM, Bernstein JA. Guideline of chronic urticaria beyond. Allergy Asthma Immunol Res. 2016; 8:396–403.13. Cicardi M, Bork K, Caballero T, Craig T, Li HH, Longhurst H, et al. Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy. 2012; 67:147–157.14. Lee SY, Kang HR, Jung JW, Jang GC, Lee SY, Ahn Y, et al. Clinical experience in managing patients with hereditary angioedema in Korea: questionnaire survey and a literature review. Allergy Asthma Respir Dis. 2014; 2:277–284.15. Rijavec M, Korošec P, Šilar M, Zidarn M, Miljković J, Košnik M. Hereditary angioedema nationwide study in Slovenia reveals four novel mutations in SERPING1 gene. PLoS One. 2013; 8:e56712.16. Waytes AT, Rosen FS, Frank MM. Treatment of hereditary angioedema with a vapor-heated C1 inhibitor concentrate. N Engl J Med. 1996; 334:1630–1634.17. Zuraw BL. Hereditary angiodema: a current state-of-the-art review, IV: short- and long-term treatment of hereditary angioedema: out with the old and in with the new. Ann Allergy Asthma Immunol. 2008; 100:S13–S18.