Investig Magn Reson Imaging.
2016 Sep;20(3):158-166. 10.13104/imri.2016.20.3.158.
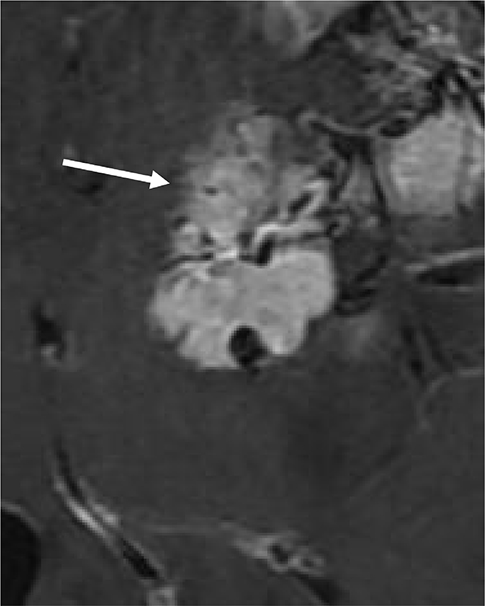
The Importance of Interface Irregularity between the Tumor and Brain Parenchyma in Differentiating between Typical and Atypical Meningiomas: Correlation with Pathology
- Affiliations
-
- 1Department of Radiology, Seoul St. Mary's Hospital, The Catholic University of Korea, Korea. ahn-kj@catholic.ac.kr
- 2Department of Pathology, Seoul St. Mary's Hospital, The Catholic University of Korea, Korea.
- 3Department of Neurosurgery, Seoul St. Mary's Hospital, The Catholic University of Korea, Korea.
- KMID: 2354796
- DOI: http://doi.org/10.13104/imri.2016.20.3.158
Abstract
- PURPOSE
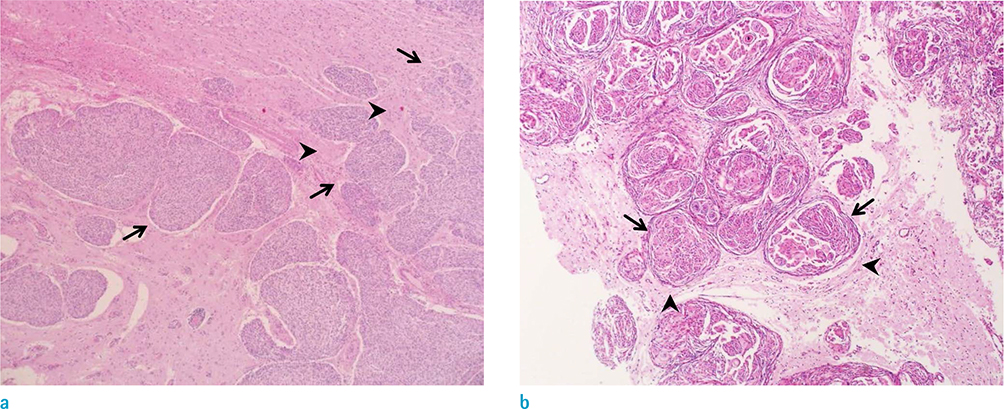
To understand clinical significance of irregular interface between meningioma and adjacent brain parenchyma in predicting histological grading of tumor, focusing on brain parenchymal invasion.
MATERIALS AND METHODS
Pathologically confirmed 79 cases with meningiomas with pathological reports about the presence of parenchymal invasion were included. We defined the presence of interface irregularity as either spiculations or fuzzy margins between the tumor and brain parenchyma. We counted number of spiculations and measured ratio of fuzzy margin length to whole length of mass with consensus of two neuroradiologists. We classified the patients into Present group and Absent group, and the two groups were compared by using the Mann-Whitney U test. Statistical correlations between the presence of an interface irregularity and brain parenchymal invasion by the tumor as well as meningioma histological grade were tested with chi-square test. The optimal cutoff values of spiculation numbers and the ratio of fuzzy margins were determined. The sensitivity and specificity of number of spiculations, ratio of fuzzy margin and the presence of irregular interface as combined parameters for predicting the parenchymal invasion were calculated using ROC curve analysis.
RESULTS
Statistically significant differences were noted between the Present and Absent groups for number of spiculations and ratio of fuzzy margin (P = 0.038 and P = 0.028, respectively). The optimal cutoff value for number of spiculations (> 4.5 with 61.1% sensitivity and 68.9% specificity) and the ratio of fuzzy margin (> 0.24 with 66.7% sensitivity and 65.6% specificity) were determined. The sensitivity and specificity of interface irregularity as the combined parameters were 72% and 59%, respectively. The interface irregularity between tumor and brain parenchyma significantly correlated with not only brain parenchymal invasion (P = 0.001) and but also histological grade (P < 0.001).
CONCLUSION
The interface irregularity between tumor and brain parenchyma in MRI can be a strong predictive factor for brain parenchymal invasion and high grade meningioma.
MeSH Terms
Figure
Reference
-
1. Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. 2005; 57:1088–1095.2. Goyal LK, Suh JH, Mohan DS, Prayson RA, Lee J, Barnett GH. Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys. 2000; 46:57–61.3. Park HJ, Kang HC, Kim IH, et al. The role of adjuvant radiotherapy in atypical meningioma. J Neurooncol. 2013; 115:241–247.4. Sanverdi SE, Ozgen B, Oguz KK, et al. Is diffusionweighted imaging useful in grading and differentiating histopathological subtypes of meningiomas? Eur J Radiol. 2012; 81:2389–2395.5. Tan LA, Boco T, Johnson AK, et al. Magnetic resonance imaging characteristics of typical and atypical/anaplastic meningiomas - Case series and literature review. Br J Neurosurg. 2014; 1–5.6. Kawahara Y, Nakada M, Hayashi Y, et al. Prediction of high-grade meningioma by preoperative MRI assessment. J Neurooncol. 2012; 108:147–152.7. Lin BJ, Chou KN, Kao HW, et al. Correlation between magnetic resonance imaging grading and pathological grading in meningioma. J Neurosurg. 2014; 121:1201–1208.8. Hsu CC, Pai CY, Kao HW, Hsueh CJ, Hsu WL, Lo CP. Do aggressive imaging features correlate with advanced histopathological grade in meningiomas? J Clin Neurosci. 2010; 17:584–587.9. Kleihues P, Sobin LH. World Health Organization classification of tumors. Cancer. 2000; 88:2887.10. Perry A, Louis DN, Scheithauer BW, Budka H, von Deimling A. Meningiomas. In : Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. World Health Organization classification of tumours of the central nervous system. 4th ed. Lyon, France: IARC Press;2007. p. 164–172.11. Essig M, Anzalone N, Combs SE, et al. MR imaging of neoplastic central nervous system lesions: review and recommendations for current practice. AJNR Am J Neuroradiol. 2012; 33:803–817.12. Filippi CG, Edgar MA, Ulug AM, Prowda JC, Heier LA, Zimmerman RD. Appearance of meningiomas on diffusion-weighted images: correlating diffusion constants with histopathologic findings. AJNR Am J Neuroradiol. 2001; 22:65–72.13. Nagar VA, Ye JR, Ng WH, et al. Diffusion-weighted MR imaging: diagnosing atypical or malignant meningiomas and detecting tumor dedifferentiation. AJNR Am J Neuroradiol. 2008; 29:1147–1152.14. Voultsinou D, Matis GK, Chrysou OI, Birbilis TA, Cheva A, Geroukis T. An atypical meningioma demystified and advanced magnetic resonance imaging techniques. J Cancer Res Ther. 2014; 10:387–389.15. Demir MK, Iplikcioglu AC, Dincer A, Arslan M, Sav A. Single voxel proton MR spectroscopy findings of typical and atypical intracranial meningiomas. Eur J Radiol. 2006; 60:48–55.16. Nakasu S, Nakasu Y, Nakajima M, Matsuda M, Handa J. Preoperative identification of meningiomas that are highly likely to recur. J Neurosurg. 1999; 90:455–462.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Atypical C-T Features of Intracranial Meningiomas and Pathological Correlation
- Correlation between Expression of p53 Protein and Prognostic Factors in Meningiomas
- Correlation of histopathologic classification with proliferative activity and DNA ploidy in 120 intracranial meningiomas, with special reference to atypical meningioma
- Role of p53 gene mutation in tumor aggressiveness of intracranial meningiomas
- Loss of Heterozygosity at 1p, 7q, 17p, and 22q in Meningiomas