J Clin Neurol.
2016 Jul;12(3):282-288. 10.3988/jcn.2016.12.3.282.
Clinical Profiles and Short-Term Outcomes of Acute Disseminated Encephalomyelitis in Adult Chinese Patients
- Affiliations
-
- 1Department of Neurology, Henan Provincial People's Hospital of Zhengzhou University, Zhengzhou, China. liwei71@126.com, majj.1124@yahoo.com.cn
- 2Department of Pharmacology, Zhengzhou Maternal and Child Health Hospital, Zhengzhou, China.
- 3Department of Neurology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China.
- 4Department of Radiology, Henan Provincial People's Hospital of Zhengzhou University, Zhengzhou, China.
- KMID: 2354114
- DOI: http://doi.org/10.3988/jcn.2016.12.3.282
Abstract
- BACKGROUND AND PURPOSE
Acute disseminated encephalomyelitis (ADEM) is an inflammatory demyelinating disorder that predominantly affects children. Previous studies have mostly involved children in Western developed countries.
METHODS
This study retrospectively reviewed the clinical profiles of ADEM in adult Chinese patients.
RESULTS
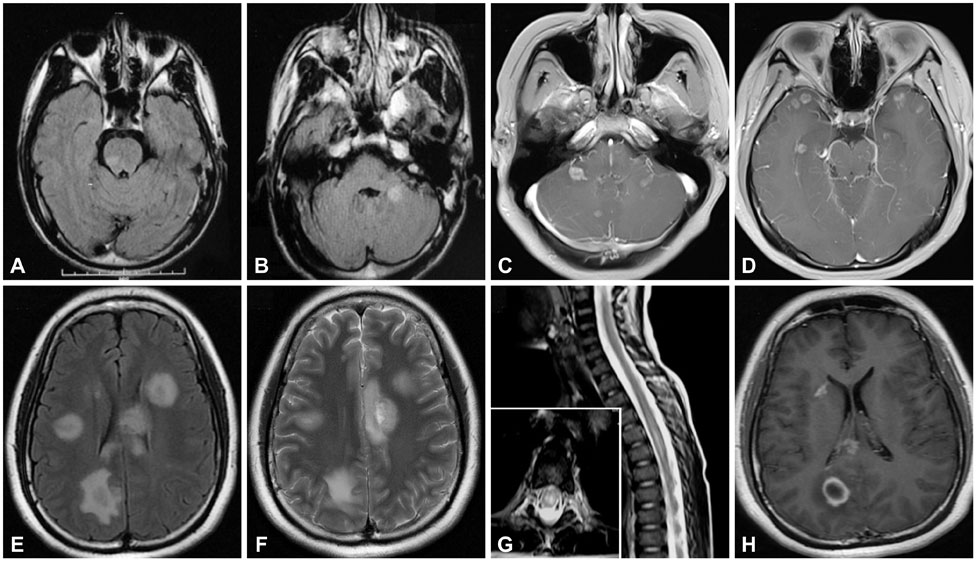
ADEM occurred during summer and autumn in about two-thirds of the 42 included patients. Prior infection was found in five patients and no preimmunization was recorded. The most frequent clinical presentations were alterations in consciousness (79%) and behavior changes (69%), followed by motor deficits (64%) and fever (50%). About one-quarter (26%) of the patients showed positive results for oligoclonal bands, and about half of them exhibited increases in the IgG index and 24-hour IgG synthesis rate. Magnetic resonance imaging showed white- and gray-matter lesions in 83% and 23% of the patients, respectively. Steroids were the main treatment, and full recovery occurred in 62% of the patients, with residual focal neurological deficits recorded in a few patients. After a mean follow-up period of 3.4 years, two patients exhibited recurrence and one patient exhibited a multiphasic course. One patient was diagnosed with multiple sclerosis (MS).
CONCLUSIONS
With the exception of the seasonal distribution pattern and prior vaccine rate, the clinical profiles of ADEM in adult Chinese patients are similar to those in pediatric populations. No specific markers are available for distinguishing ADEM from MS at the initial presentation. Careful clinical evaluations, cerebrospinal fluid measurements, and neuroradiological examinations with long-term follow-up will aid the correct diagnosis of ADEM.
Keyword
MeSH Terms
-
Adult*
Asian Continental Ancestry Group*
Cerebrospinal Fluid
Child
Consciousness
Demyelinating Diseases
Developed Countries
Diagnosis
Encephalomyelitis, Acute Disseminated*
Fever
Follow-Up Studies
Humans
Immunoglobulin G
Magnetic Resonance Imaging
Multiple Sclerosis
Oligoclonal Bands
Recurrence
Retrospective Studies
Seasons
Steroids
Immunoglobulin G
Oligoclonal Bands
Steroids
Figure
Reference
-
1. Tenembaum S, Chitnis T, Ness J, Hahn JS;. Acute disseminated encephalomyelitis. Neurology. 2007; 68:16 Suppl 2. S23–S36.
Article2. Dale RC, de Sousa C, Chong WK, Cox TC, Harding B, Neville BG. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000; 123(Pt 12):2407–2422.
Article3. Chen S, Wu A, Zhang B, Li J, Zhang L, Lin Y, et al. A case of exacerbated multiphasic disseminated encephalomyelitis after interferon β treatment. J Neurol Sci. 2013; 325:176–179.
Article4. Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002; 59:1224–1231.
Article5. Schwarz S, Mohr A, Knauth M, Wildemann B, Storch-Hagenlocher B. Acute disseminated encephalomyelitis: a follow-up study of 40 adult patients. Neurology. 2001; 56:1313–1318.
Article6. Krupp LB, Banwell B, Tenembaum S;. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007; 68:16 Suppl 2. S7–S12.
Article7. Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005; 58:840–846.
Article8. Leake JA, Albani S, Kao AS, Senac MO, Billman GF, Nespeca MP, et al. Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatr Infect Dis J. 2004; 23:756–764.
Article9. Murthy SN, Faden HS, Cohen ME, Bakshi R. Acute disseminated encephalomyelitis in children. Pediatrics. 2002; 110(2 Pt 1):e21.
Article10. Idrissova ZhR, Boldyreva MN, Dekonenko EP, Malishev NA, Leontyeva IY, Martinenko IN, et al. Acute disseminated encephalomyelitis in children: clinical features and HLA-DR linkage. Eur J Neurol. 2003; 10:537–546.
Article11. Hynson JL, Kornberg AJ, Coleman LT, Shield L, Harvey AS, Kean MJ. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology. 2001; 56:1308–1312.
Article12. Likasitwattanakul S, Chiewvit P. Acute disseminated encephalomyelitis in Siriraj Hospital: clinical manifestations and short-term outcome. J Med Assoc Thai. 2012; 95:391–396.13. Lu Z, Zhang B, Qiu W, Kang Z, Shen L, Long Y, et al. Comparative brain stem lesions on MRI of acute disseminated encephalomyelitis, neuromyelitis optica, and multiple sclerosis. PLoS One. 2011; 6:e22766.
Article14. Neuteboom RF, Boon M, Catsman Berrevoets CE, Vles JS, Gooskens RH, Stroink H, et al. Prognostic factors after a first attack of inflammatory CNS demyelination in children. Neurology. 2008; 71:967–973.
Article15. Pohl D, Rostasy K, Reiber H, Hanefeld F. CSF characteristics in early-onset multiple sclerosis. Neurology. 2004; 63:1966–1967.
Article16. Sharief MK, Thompson EJ. The predictive value of intrathecal immunoglobulin synthesis and magnetic resonance imaging in acute isolated syndromes for subsequent development of multiple sclerosis. Ann Neurol. 1991; 29:147–151.
Article17. Mikaeloff Y, Suissa S, Vallée L, Lubetzki C, Ponsot G, Confavreux C, et al. First episode of acute CNS inflammatory demyelination in childhood: prognostic factors for multiple sclerosis and disability. J Pediatr. 2004; 144:246–252.
Article18. Callen DJ, Shroff MM, Branson HM, Li DK, Lotze T, Stephens D, et al. Role of MRI in the differentiation of ADEM from MS in children. Neurology. 2009; 72:968–973.
Article19. Ketelslegers IA, Neuteboom RF, Boon M, Catsman-Berrevoets CE, Hintzen RQ;. A comparison of MRI criteria for diagnosing pediatric ADEM and MS. Neurology. 2010; 74:1412–1415.
Article20. Garg RK. Acute disseminated encephalomyelitis. Postgrad Med J. 2003; 79:11–17.
Article21. Liu JG, Qiao WY, Dong QW, Zhang HL, Zheng KH, Qian HR, et al. [Clinical features and neuroimaging findings of 12 patients with acute disseminated encephalomyelitis involved in corpus callosum]. Zhonghua Yi Xue Za Zhi. 2012; 92:3036–3041.22. Giorgio A, Battaglini M, Rocca MA, De Leucio A, Absinta M, van Schijndel R, et al. Location of brain lesions predicts conversion of clinically isolated syndromes to multiple sclerosis. Neurology. 2013; 80:234–241.
Article23. Ikeda K, Ito H, Hidaka T, Takazawa T, Sekine T, Yoshii Y, et al. Repeated non-enhancing tumefactive lesions in a patient with a neuromyelitis optica spectrum disorder. Intern Med. 2011; 50:1061–1064.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute disseminated encephalomyelitis
- A case of acute disseminated encephalomyelitis
- Acute Disseminated Encephalomyelitis Following Pneumococcal Vaccination
- Acute Disseminated Encephalomyelitis Associated with Influenza Vaccination
- A Case of Multiple Sclerosis Presenting as Acute Disseminated Encephalomyelitis