Blood Res.
2016 Sep;51(3):187-192. 10.5045/br.2016.51.3.187.
Treatment outcomes of IMEP as a front-line chemotherapy for patients with peripheral T-cell lymphomas
- Affiliations
-
- 1Department of Internal Medicine, Hemato-Oncology, Inje University Busan Paik Hospital, Busan, Korea. wonsik112@gmail.com
- 2Department of Internal Medicine, Hemato-Oncology, Inje University Haeundae Paik Hospital, Busan, Korea.
- KMID: 2353500
- DOI: http://doi.org/10.5045/br.2016.51.3.187
Abstract
- BACKGROUND
This study aimed to assess the treatment outcomes of ifosphamide, mesna, etoposide, and prednisolone (IMEP) combination regimen as a front-line chemotherapy in patients with peripheral T-cell lymphomas (PTCLs).
METHODS
Clinical data of 38 newly diagnosed PTCLs patients who underwent IMEP at Busan Paik Hospital from January 2002 to December 2013 were retrospectively analyzed.
RESULTS
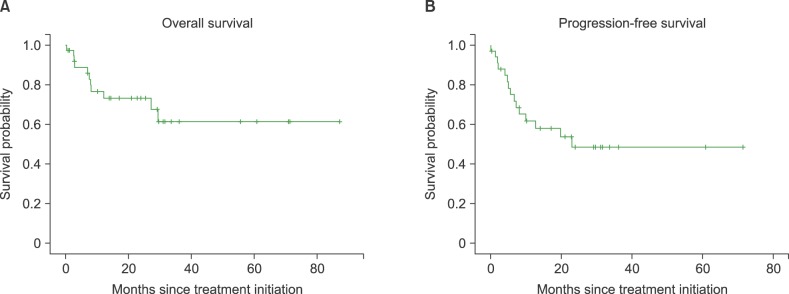
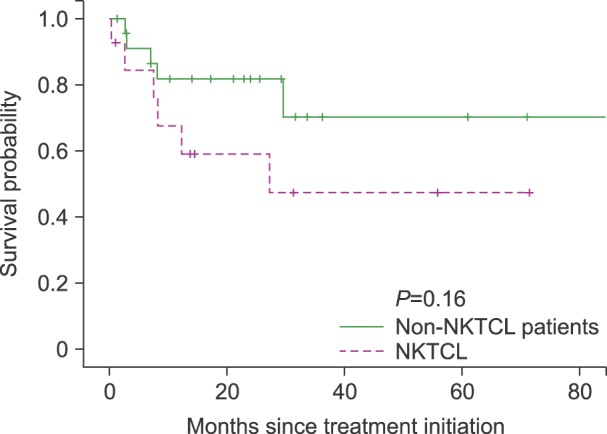
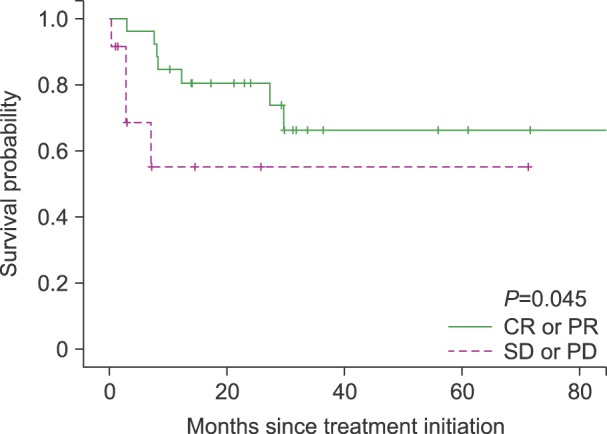
The overall response rate was 68.5%, with 21 (55.3%) complete response/complete response unconfirmed and 6 (15.8%) partial response (PR). The median follow-up duration was 25.5 months (range, 0.2-87.3). The median overall survival was not reached and 2-year survival rate was 67%. The median progression free survival was 23 months. The most frequently reported adverse effects higher than grade 3 were hematologic toxicities including neutropenia (68.4%), thrombocytopenia (42.1%). There was no treatment-related mortality.
CONCLUSION
IMEP regimen is effective and safe as a front-line chemotherapy in patients with PTCLs.
Keyword
MeSH Terms
Figure
Reference
-
1. Armitage JO. The aggressive peripheral T-cell lymphomas: 2013. Am J Hematol. 2013; 88:910–918. PMID: 24078271.
Article2. Kim JM, Ko YH, Lee SS, et al. WHO classification of malignant lymphomas in Korea: Report of the third nationwide study. Korean J Pathol. 2011; 45:254–260.
Article3. Vose J, Armitage J, Weisenburger D. International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol. 2008; 26:4124–4130. PMID: 18626005.4. Gisselbrecht C, Gaulard P, Lepage E, et al. Groupe d'etudes des lymphomes de l'adulte (GELA). Prognostic significance of T-cell phenotype in aggressive non-hodgkin's lymphomas. Blood. 1998; 92:76–82. PMID: 9639502.5. Cabanillas F, Hagemeister FB, Bodey GP, Freireich EJ. IMVP-16: An effective regimen for patients with lymphoma who have relapsed after initial combination chemotherapy. Blood. 1982; 60:693–697. PMID: 7104493.
Article6. Huijgens PC, Ossenkoppele GJ, Van Der Lelie J, Thomas LL, Wijngaarden MJ, Slaper CM. IMVP-16 followed by high dose chemotherapy and autologous bone marrow transplantation as salvage treatment for malignant lymphoma. Hematol Oncol. 1991; 9:245–251. PMID: 1743627.
Article7. Aurer I, Duraković N, Radman I, et al. Combination of ifosfamide, methotrexate, and etoposide (IMVP) as a salvage therapy for relapsed and refractory aggressive non-Hodgkin lymphoma: Retrospective study. Croat Med J. 2002; 43:550–554. PMID: 12402394.8. Cheson BD, Horning SJ, Coiffier B, et al. NCI Sponsored International Working Group. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol. 1999; 17:1244. PMID: 10561185.
Article9. Simon A, Peoch M, Casassus P, et al. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS-LTP95. Br J Haematol. 2010; 151:159–166. PMID: 20738307.
Article10. Lee KW, Yun T, Kim DW, et al. First-line ifosfamide, methotrexate, etoposide and prednisolone chemotherapy +/- radiotherapy is active in stage I/II extranodal NK/T-cell lymphoma. Leuk Lymphoma. 2006; 47:1274–1282. PMID: 16923557.
Article11. Lee KW, Kim DW, Im SA, et al. Efficacy of ifosfamide, methotrexate, etoposide and prednisone (IMEP) chemotherapy in extranodal NK/T-cell lymphoma, nasal type. J Clin Oncol (ASCO Annual Meeting Abstracts). 2004; 22(Suppl):abst 6685.
Article12. Kim TM, Kim DW, Kang YK, et al. A phase II study of ifosfamide, methotrexate, etoposide, and prednisolone for previously untreated stage I/II extranodal natural killer/T-cell lymphoma, nasal type: A multicenter trial of the Korean Cancer Study Group. Oncologist. 2014; 19:1129–1130. PMID: 25280488.
Article13. Park BB, Kim WS, Lee J, et al. IMVP-16/Pd followed by high-dose chemotherapy and autologous stem cell transplantation as a salvage therapy for refractory or relapsed peripheral T-cell lymphomas. Leuk Lymphoma. 2005; 46:1743–1748. PMID: 16263576.
Article14. Kim M, Kim TM, Kim KH, et al. Ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) plus L-asparaginase as a first-line therapy improves outcomes in stage III/IV NK/T cell-lymphoma, nasal type (NTCL). Ann Hematol. 2015; 94:437–444. PMID: 25300500.
Article15. Ohmachi K, Tobinai K, Kobayashi Y, et al. Phase III trial of CHOP-21 versus CHOP-14 for aggressive non-Hodgkin's lymphoma: Final results of the Japan Clinical Oncology Group Study, JCOG 9809. Ann Oncol. 2011; 22:1382–1391. PMID: 21196441.
Article16. Pfreundschuh M, Trumper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: Results of the NHL-B1 trial of the DSHNHL. Blood. 2004; 104:626–633. PMID: 14982884.
Article17. Wöhrer S, Chott A, Drach J, et al. Chemotherapy with cyclophosphamide, doxorubicin, etoposide, vincristine and prednisone (CHOEP) is not effective in patients with enteropathy-type intestinal T-cell lymphoma. Ann Oncol. 2004; 15:1680–1683. PMID: 15520071.
Article18. Kim JG, Sohn SK, Chae YS, et al. CHOP plus etoposide and gemcitabine (CHOP-EG) as front-line chemotherapy for patients with peripheral T cell lymphomas. Cancer Chemother Pharmacol. 2006; 58:35–39. PMID: 16308699.
Article19. Sung HJ, Kim SJ, Seo HY, et al. Prospective analysis of treatment outcome and prognostic factors in patients with T-cell lymphomas treated by CEOP-B: Single institutional study. Br J Haematol. 2006; 134:45–53. PMID: 16803566.
Article20. Escalón MP, Liu NS, Yang Y, et al. Prognostic factors and treatment of patients with T-cell non-Hodgkin lymphoma: The M. D. Anderson Cancer Center experience. Cancer. 2005; 103:2091–2098. PMID: 15816054.21. Morabito F, Gallamini A, Stelitano C, et al. Clinical relevance of immunophenotype in a retrospective comparative study of 297 peripheral T-cell lymphomas, unspecified, and 496 diffuse large B-cell lymphomas: Experience of the Intergruppo Italiano Linformi. Cancer. 2004; 101:1601–1608. PMID: 15378507.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of peripheral T-cell lymphoma with central nervous system and bilateral pulmonary involvement
- Gastrointestinal Tract Lymphoma
- A Case of a Synchronous Peripheral T-cell Lymphoma in a Patient with Systemic Lupus Erythematosus
- Development of Internet-based Medical Educational Program
- Helicobacter pylori-negative Gastric Mucosa-associated Lymphoid Tissue Lymphoma