J Breast Cancer.
2014 Mar;17(1):33-39.
Serial Serum HER2 Measurements for the Detection of Breast Cancer Recurrence in HER2-Positive Patients
- Affiliations
-
- 1Department of Surgery, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea. hyunah@kcch.re.kr
- 2Department of Pathology, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- 3Department of Laboratory Medicine, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- 4Division of Radiation Cancer Research, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
Abstract
- PURPOSE
The measurement of serum human epidermal growth factor receptor 2 (HER2) extracellular domain levels is a well-established method for evaluating whether a metastatic HER2-positive breast cancer patient will respond to HER2-targeted treatment. However, little is known about the value of serum HER2 for detecting disease relapse following curative surgical treatment in breast cancer patients. The purpose of this study was to evaluate the sensitivity of serum HER2, carcinoembryonic antigen (CEA), and carcinoma antigen 15-3 (CA 15-3) for the detection of disease recurrence in postoperative breast cancer patients with a primary HER2-positive tumor.
METHODS
Serial measurements were taken of serum HER2, CEA, and CA 15-3 levels in patients with primary invasive HER2-positive breast cancer who underwent curative surgical treatment between January 2008 and December 2010. Following treatment, serum HER2 levels were monitored every 6 months using a chemiluminescence immunoassay.
RESULTS
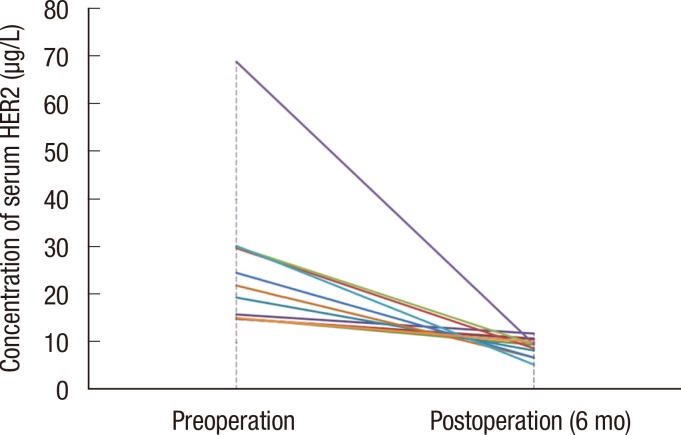
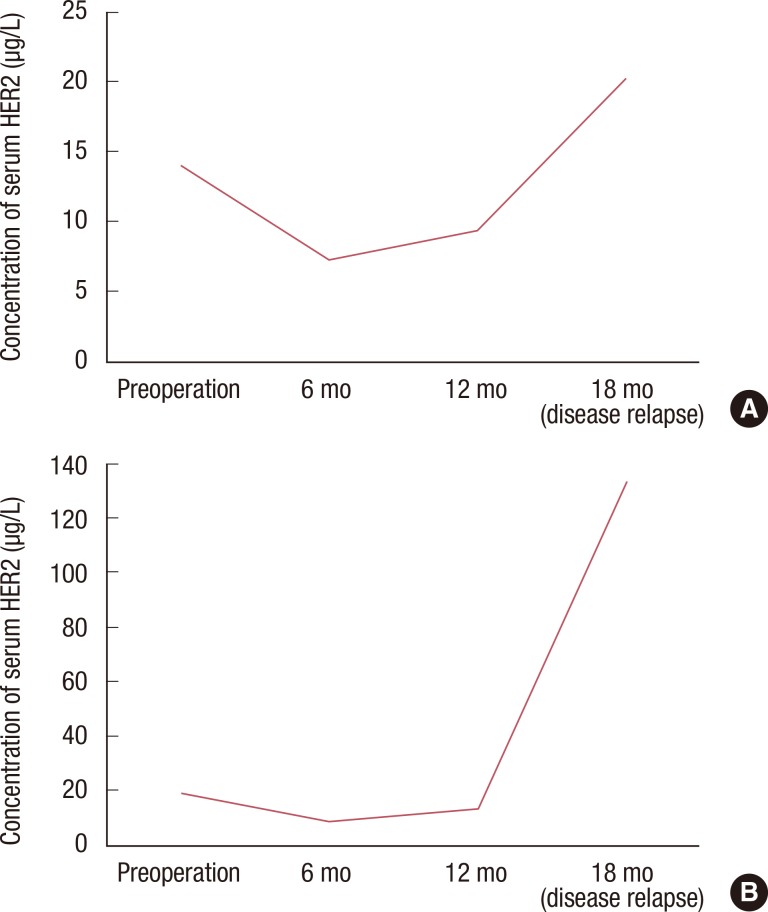
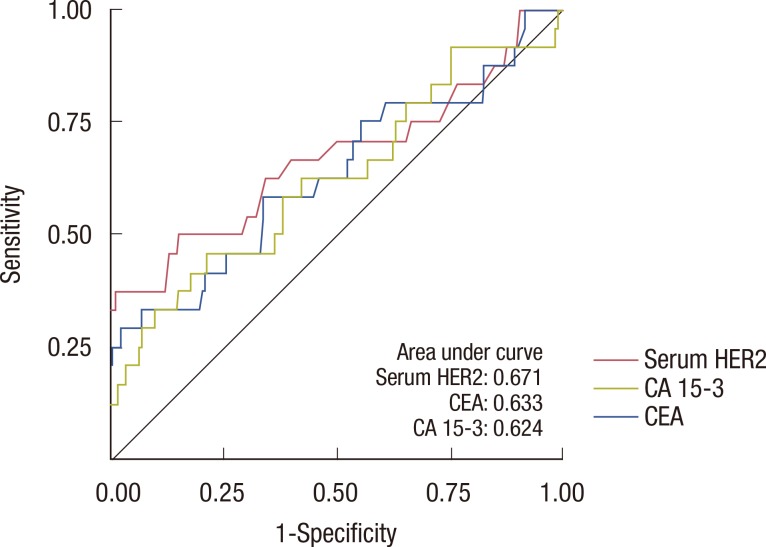
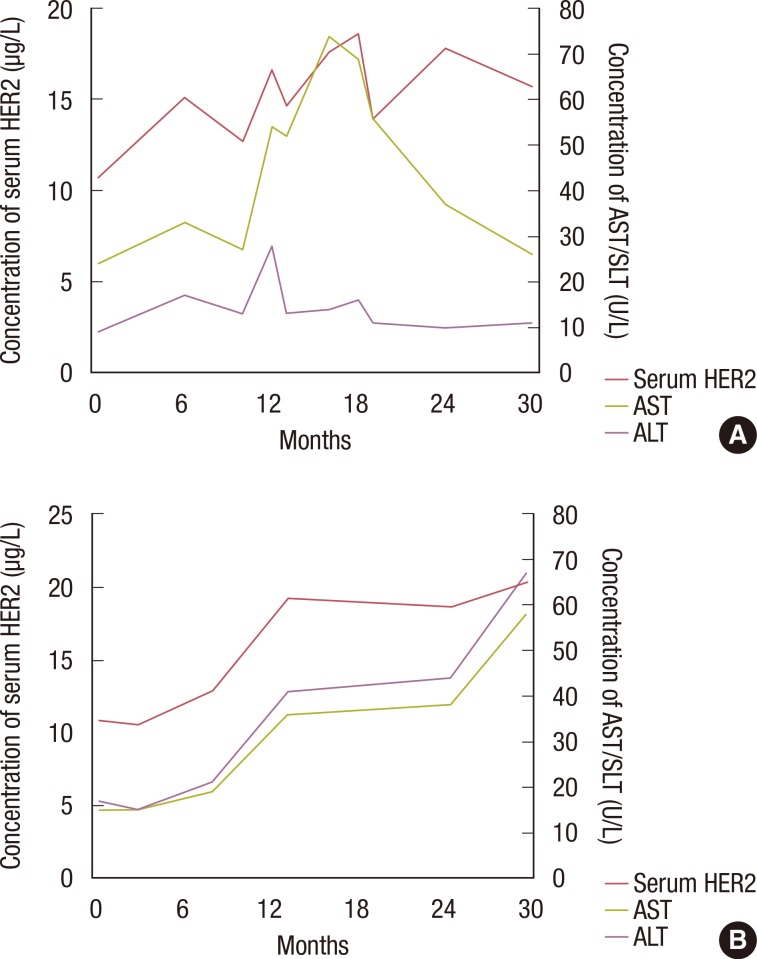
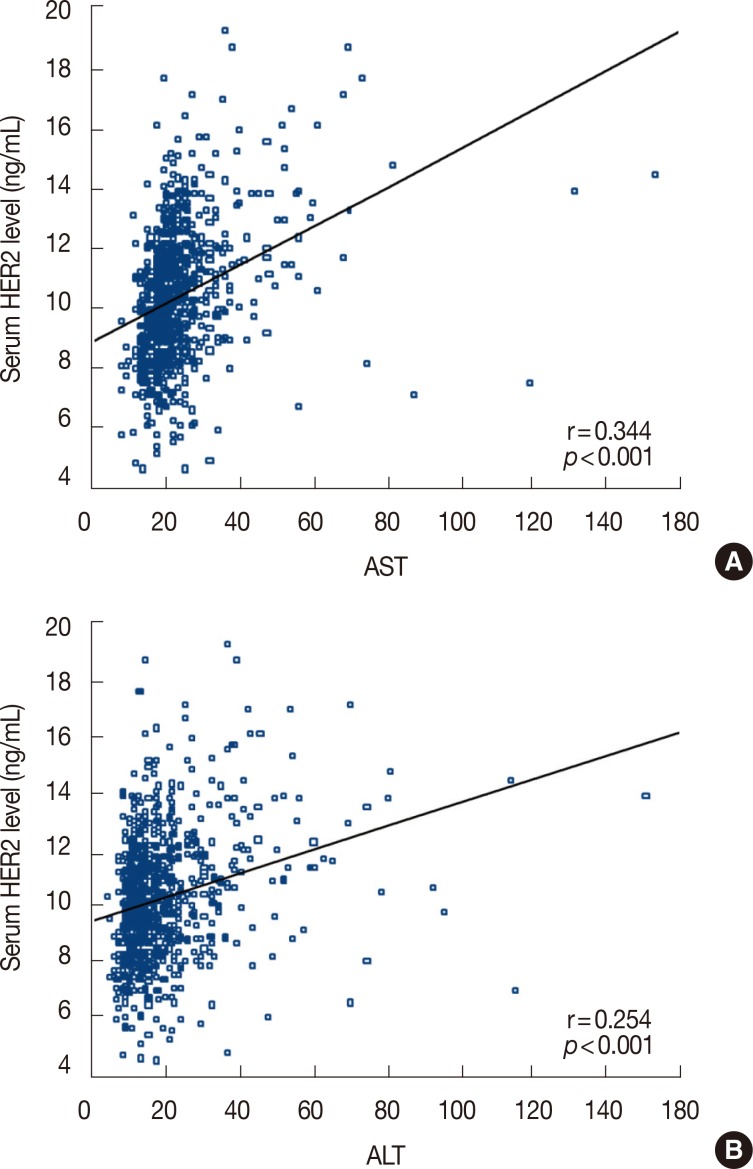
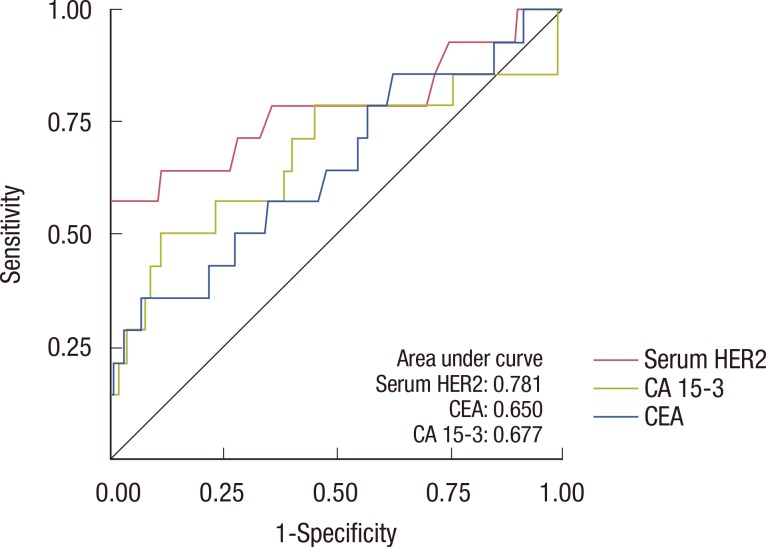
Overall, 264 patients were analyzed in this retrospective study. The median follow-up period was 27.7 months, and 24 patients relapsed during follow-up. The sensitivity of serum HER2, CEA, and CA 15-3 for the detection of disease recurrence was 37.5%, 25.1%, and 12.5%, respectively. Sensitivity increased to 45.8% when all three tumor markers were combined in the analysis. In a subgroup of patients without liver disease, the sensitivity of serum HER2, CEA, and CA 15-3 was 57.1%, 21.4%, and 14.3%, respectively. Of the 264 patients in this study, 80 patients had chronic hepatitis, liver cirrhosis, or abnormal aspartate aminotransferase or alanine aminotransferase levels during the follow-up period. Following the exclusion of these patients, the sensitivity of serum HER2 for the detection of disease recurrence increased to 57.1%.
CONCLUSION
Serial serum HER2 measurement may be useful for the detection of disease relapse in patients with HER2-positive breast cancer. Abnormal liver function can result in elevated serum HER2 in the absence of disease recurrence.
Keyword
MeSH Terms
-
Alanine Transaminase
Aspartate Aminotransferases
Breast Neoplasms*
Breast*
Carcinoembryonic Antigen
Follow-Up Studies
Hepatitis, Chronic
Humans
Immunoassay
Liver
Liver Cirrhosis
Liver Diseases
Luminescence
Receptor, Epidermal Growth Factor
Recurrence*
Retrospective Studies
Biomarkers, Tumor
Alanine Transaminase
Aspartate Aminotransferases
Carcinoembryonic Antigen
Receptor, Epidermal Growth Factor
Figure
Reference
-
1. Baxevanis CN, Sotiropoulou PA, Sotiriadou NN, Papamichail M. Immunobiology of HER-2/neu oncoprotein and its potential application in cancer immunotherapy. Cancer Immunol Immunother. 2004; 53:166–175. PMID: 14685781.2. Ryu DW, Lee CH. Impact of serum HER2 levels on survival and its correlation with clinicopathological parameters in women with breast cancer. J Breast Cancer. 2012; 15:71–78. PMID: 22493631.
Article3. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987; 235:177–182. PMID: 3798106.4. Andrulis IL, Bull SB, Blackstein ME, Sutherland D, Mak C, Sidlofsky S, et al. Toronto Breast Cancer Study Group. neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. J Clin Oncol. 1998; 16:1340–1349. PMID: 9552035.5. Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006; 3:269–280. PMID: 16683005.
Article6. Duffy MJ. Serum tumor markers in breast cancer: are they of clinical value? Clin Chem. 2006; 52:345–351. PMID: 16410341.
Article7. Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007; 25:5287–5312. PMID: 17954709.
Article8. Baskić D, Ristić P, Matić S, Banković D, Popović S, Arsenijević N. Clinical evaluation of the simultaneous determination of CA 15-3, CA 125 and sHER2 in breast cancer. Biomarkers. 2007; 12:657–667. PMID: 17852076.
Article9. Fehm T, Jäger W, Krämer S, Sohn C, Solomayer E, Wallwiener D, et al. Prognostic significance of serum HER2 and CA 15-3 at the time of diagnosis of metastatic breast cancer. Anticancer Res. 2004; 24(3B):1987–1992. PMID: 15274389.10. Cook GB, Neaman IE, Goldblatt JL, Cambetas DR, Hussain M, Lüftner D, et al. Clinical utility of serum HER-2/neu testing on the Bayer Immuno 1 automated system in breast cancer. Anticancer Res. 2001; 21(2B):1465–1470. PMID: 11396233.11. Schwartz MK, Smith C, Schwartz DC, Dnistrian A, Neiman I. Monitoring therapy by serum HER-2/neu. Int J Biol Markers. 2000; 15:324–329. PMID: 11192828.
Article12. Gerhardt W, Keller H. Evaluation of test data from clinical studies. I. Terminology, graphic interpretation, diagnostic strategies, and selection of sample groups. II. Critical review of the concepts of efficiency, receiver operated characteristics (ROC), and likelihood ratios. Scand J Clin Lab Invest Suppl. 1986; 181:1–74. PMID: 3460162.13. Molina R, Zanón G, Filella X, Moreno F, Jo J, Daniels M, et al. Use of serial carcinoembryonic antigen and CA 15.3 assays in detecting relapses in breast cancer patients. Breast Cancer Res Treat. 1995; 36:41–48. PMID: 7579505.
Article14. Pedersen AC, Sørensen PD, Jacobsen EH, Madsen JS, Brandslund I. Sensitivity of CA 15-3, CEA and serum HER2 in the early detection of recurrence of breast cancer. Clin Chem Lab Med. 2013; 51:1511–1519. PMID: 23403727.
Article15. Sugano K, Ushiama M, Fukutomi T, Tsuda H, Kitoh T, Ohkura H. Combined measurement of the c-erbB-2 protein in breast carcinoma tissues and sera is useful as a sensitive tumor marker for monitoring tumor relapse. Int J Cancer. 2000; 89:329–336. PMID: 10956406.
Article16. Molina R, Jo J, Filella X, Zanón G, Farrus B, Muñoz M, et al. C-erbB-2, CEA and CA 15.3 serum levels in the early diagnosis of recurrence of breast cancer patients. Anticancer Res. 1999; 19(4A):2551–2555. PMID: 10470193.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and Treatment of HER2-Positive Breast Cancer
- Serum HER2 as a Response Indicator to Various Chemotherapeutic Agents in Tissue HER2 Positive Metastatic Breast Cancer
- Impact of HER2-Low Status on Pathologic Complete Response and Survival Outcome Among Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy
- Impact of Serum HER2 Levels on Survival and Its Correlation with Clinicopathological Parameters in Women with Breast Cancer
- Prognostic Value of the Evolution of HER2-Low Expression after Neoadjuvant Chemotherapy