J Breast Cancer.
2014 Jun;17(2):136-142.
Fatty Acid Composition of Tissue Cultured Breast Carcinoma and the Effect of Stearoyl-CoA Desaturase 1 Inhibition
- Affiliations
-
- 1Department of Biochemistry and Clinical Laboratories, Tabriz University of Medical Sciences School of Medicine, Tabriz, Iran.
- 2Biotechnology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. darabim@tbzmed.ac.ir
- 3Department of Thoracic Surgery, Tabriz University of Medical Sciences, Tabriz, Iran.
- 4Students Research Committee, Department of Anatomy and Cell Biology, Shahid Beheshti University of Medical Sciences School of Medicine, Tehran, Iran.
- 5Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
- 6Liver and Gastrointestinal Disease Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Abstract
- PURPOSE
Stearoyl-CoA desaturase 1 (SCD1) is a novel therapeutic target in various malignancies, including breast cancer. The present study was designed to investigate the effect of the pharmacologic inhibition of SCD1 on fatty acid composition in tissue explant cultures of human breast cancer and to compare these effects with those in adjacent nonneoplastic breast tissue.
METHODS
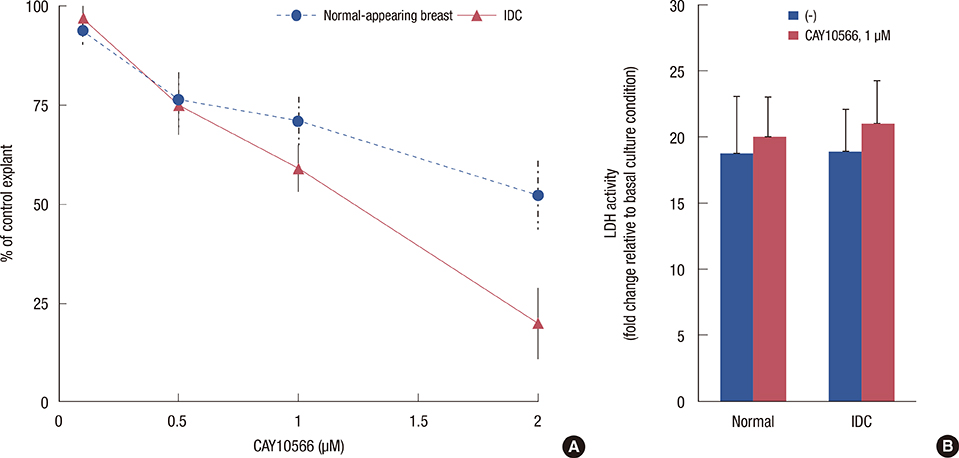
Paired samples of tumor and adjacent noncancerous tissue were isolated from 12 patients with infiltrating ductal breast cancer. Samples were explant cultured in vitro, exposed to the highly selective SCD1 inhibitor CAY10566, and examined for fatty acid composition by gas liquid chromatography. The cytotoxic and antigrowth effects were evaluated by quantification of lactate dehydrogenase release and by sulforhodamine B (SRB) measurement, respectively.
RESULTS
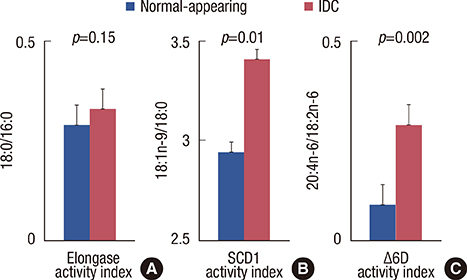
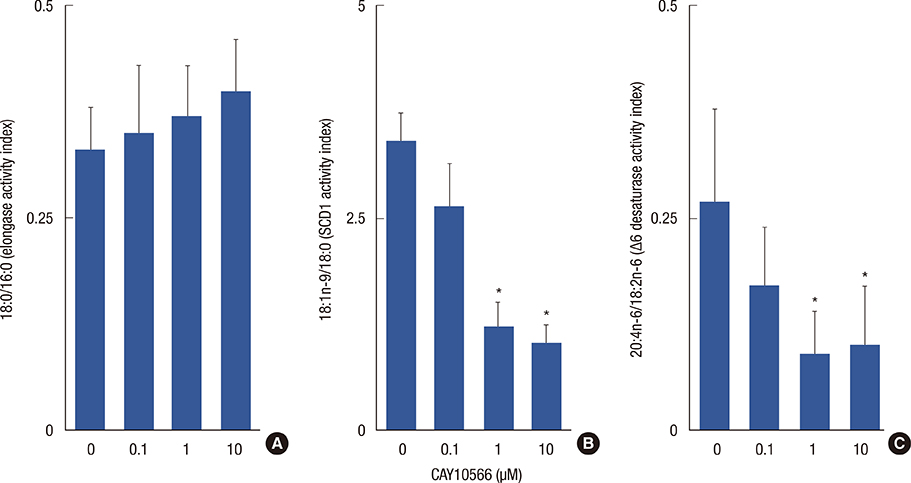
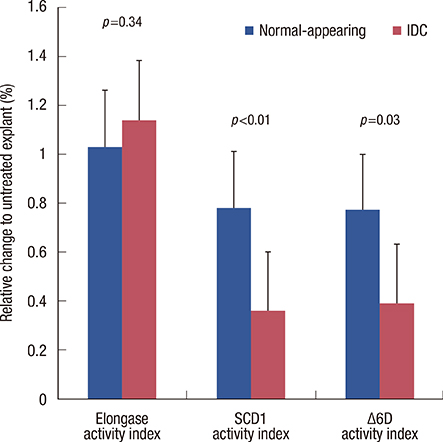
Breast cancer tissue samples were found to have higher levels of monounsaturated fatty acids (MUFA) (p<0.001) and arachidonic acid (20:4n-6, p<0.001) and a lower level of linoleic acid (18:2n-6, p=0.02) than the normal-appearing breast tissues. While exhibiting no evident cytotoxicity, treatment with the SCD1 inhibitor, CAY10566 (0.1-1 microM), for 48 hours significantly increased 18:2n-6 levels in both the tumor and adjacent normal-appearing tissue (approximately 1.2 fold, p<0.05). However, the breast cancer tissue samples showed significant increases in the levels of MUFA and 20:4n-6 compared to the normal-appearing breast tissues (p<0.05). The SRB growth assay revealed a higher rate of inhibition with the SCD1 inhibitor in breast cancer tissues than in normal-appearing tissues (p<0.01, 41% vs. 29%). The SCD1 inhibitor also elevated saturated fatty acid (1.46-fold, p=0.001) levels only in the tumor tissue explant.
CONCLUSION
The fatty acid composition and response to SCD1 inhibition differed between the explant cultures from breast cancer and the adjacent normal-appearing tissue. Altered fatty acid composition induced by SCD1 inhibition may also, in addition to Delta9 desaturation, modulate other reactions in de novo fatty acid synthesis and lipogenesis, and subsequently affect the overall survival and progression of breast cancer.
MeSH Terms
-
Arachidonic Acid
Breast
Breast Neoplasms*
Chromatography, Liquid
Fatty Acid Desaturases
Fatty Acids, Monounsaturated
Humans
L-Lactate Dehydrogenase
Linoleic Acid
Lipogenesis
Stearoyl-CoA Desaturase*
Tissue Culture Techniques
Arachidonic Acid
Fatty Acid Desaturases
Fatty Acids, Monounsaturated
L-Lactate Dehydrogenase
Linoleic Acid
Stearoyl-CoA Desaturase
Figure
Reference
-
1. Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012; 380:1840–1850.
Article2. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011; 11:85–95.
Article3. Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009; 100:1369–1372.
Article4. Roongta UV, Pabalan JG, Wang X, Ryseck RP, Fargnoli J, Henley BJ, et al. Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Mol Cancer Res. 2011; 9:1551–1561.
Article5. Li J, Ding SF, Habib NA, Fermor BF, Wood CB, Gilmour RS. Partial characterization of a cDNA for human stearoyl-CoA desaturase and changes in its mRNA expression in some normal and malignant tissues. Int J Cancer. 1994; 57:348–352.
Article6. Mason P, Liang B, Li L, Fremgen T, Murphy E, Quinn A, et al. SCD1 inhibition causes cancer cell death by depleting mono-unsaturated fatty acids. PLoS One. 2012; 7:e33823.
Article7. Scaglia N, Igal RA. Inhibition of stearoyl-CoA desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis. Int J Oncol. 2008; 33:839–850.
Article8. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–410.
Article9. Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986; 27:114–120.
Article10. Noori M, Darabi M, Rahimipour A, Rahbani M, Abadi NA, Darabi M, et al. Fatty acid composition of HDL phospholipids and coronary artery disease. J Clin Lipidol. 2009; 3:39–44.
Article11. Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006; 1:1112–1116.
Article12. Igal RA. Roles of stearoylCoA desaturase-1 in the regulation of cancer cell growth, survival and tumorigenesis. Cancers (Basel). 2011; 3:2462–2477.
Article13. Hess D, Chisholm JW, Igal RA. Inhibition of stearoylCoA desaturase activity blocks cell cycle progression and induces programmed cell death in lung cancer cells. PLoS One. 2010; 5:e11394.
Article14. Verschoor ML, Verschoor CP, Singh G. Ets-1 global gene expression profile reveals associations with metabolism and oxidative stress in ovarian and breast cancers. Cancer Metab. 2013; 1:17.
Article15. Thai SF, Allen JW, DeAngelo AB, George MH, Fuscoe JC. Detection of early gene expression changes by differential display in the livers of mice exposed to dichloroacetic acid. Carcinogenesis. 2001; 22:1317–1322.
Article16. Minville-Walz M, Pierre AS, Pichon L, Bellenger S, Fèvre C, Bellenger J, et al. Inhibition of stearoyl-CoA desaturase 1 expression induces CHOP-dependent cell death in human cancer cells. PLoS One. 2010; 5:e14363.
Article17. Azordegan N, Fraser V, Le K, Hillyer LM, Ma DW, Fischer G, et al. Carcinogenesis alters fatty acid profile in breast tissue. Mol Cell Biochem. 2013; 374:223–232.
Article18. Brown JM, Chung S, Sawyer JK, Degirolamo C, Alger HM, Nguyen T, et al. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation. 2008; 118:1467–1475.
Article19. Pender-Cudlip MC, Krag KJ, Martini D, Yu J, Guidi A, Skinner SS, et al. Delta-6-desaturase activity and arachidonic acid synthesis are increased in human breast cancer tissue. Cancer Sci. 2013; 104:760–764.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sterculic Acid and Its Analogues Are Potent Inhibitors of Toxoplasma gondii
- Fatty acid composition and delta 6 desaturase activities in strepto-zotocin induced diabetic rats following omega-3 fatty acid supple-mentation
- Carnosic Acid Inhibits Lipid Accumulation in 3T3-L1 Adipocytes Through Attenuation of Fatty Acid Desaturation
- Delta 6 desaturase activity and fatty acid composition in experimen- tal diabetic rats
- Picroside II attenuates fatty acid accumulation in HepG2 cells via modulation of fatty acid uptake and synthesis