Endocrinol Metab.
2016 Sep;31(3):469-475. 10.3803/EnM.2016.31.3.469.
Macrophage Densities Correlated with CXC Chemokine Receptor 4 Expression and Related with Poor Survival in Anaplastic Thyroid Cancer
- Affiliations
-
- 1Department of Internal Medicine, National Medical Center, Seoul, Korea. swchomd@gmail.com
- 2Department of Surgery, National Medical Center, Seoul, Korea.
- 3Department of Pathology, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea. youngakim@gmail.com
- 4Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2352988
- DOI: http://doi.org/10.3803/EnM.2016.31.3.469
Abstract
- BACKGROUND
Tumor associated macrophages (TAMs) and CXC chemokine receptor 4 (CXCR4) have emerged as potential biomarkers in various human cancers. The aims of this study were to investigate the clinical characteristics of anaplastic thyroid cancer (ATC) patients according to the TAM numbers in the tumor tissue, and to evaluate the associations between CXCR4 expressions and macrophage densities in ATC tumor microenvironment.
METHODS
Total 14 ATC samples from thyroid tissue microarray were used. Immunohistochemical staining was performed using anti-CD163 and anti-CXCR4 antibodies. According to the immunoreactivity of CD163, all subjects were divided into two groups: low-CD163 (n=8) and high-CD163 (n=6) groups.
RESULTS
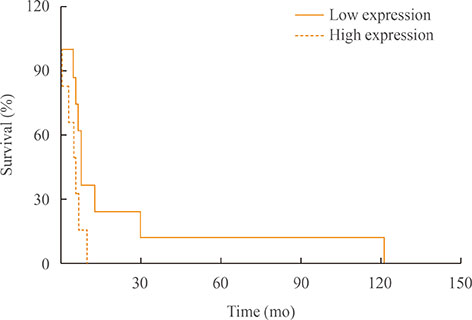
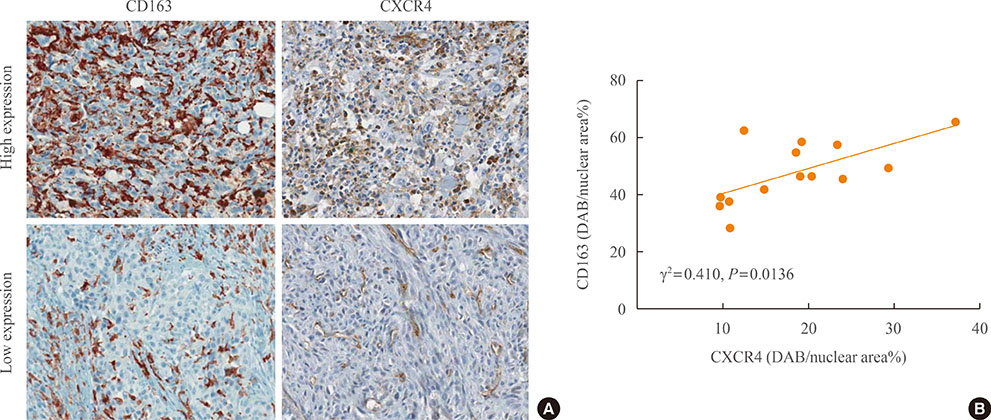
The mean diagnostic age was 65±7 years and the median tumor size was 4.3 cm, ranging 2.5 to 15 cm. Clinicopathological characteristics were not significantly different between low-CD163 and high-CD163 groups, while age of diagnosis was younger in high-CD163 group than that of low-CD163 group with marginal significance (56.9±5.5 years vs. 67.5±6.8 years, P=0.09). However, overall survival was significantly reduced in high-CD163 group (5.5 months [range, 1 to 10]) compared with low-CD163 groups (8.8 months [range, 6 to 121); log-rank test, P=0.0443). Moreover, high-CD163 group showed strong CXCR4 expressions in both cancer and stromal compartments, while low-CD163 group showed relatively weak, stromal-dominant CXCR4 expressions. Additionally, CD163 and CXCR4 expressions showed a strong positive correlation (γ²=0.432, P=0.013).
CONCLUSION
Increased number of TAMs showed poor overall survival in ATC, suggesting TAMs are potentially a prognostic biomarker for ATC. CXCR4 expression was significantly correlated with CD163-positive TAM densities, which suggest the possible role of CXCR4 in TAM recruitments.
MeSH Terms
Figure
Cited by 1 articles
-
Mouse Models as a Tool for Understanding Progression in BrafV600E-Driven Thyroid Cancers
Iñigo Landa, Jeffrey A. Knauf
Endocrinol Metab. 2019;34(1):11-22. doi: 10.3803/EnM.2019.34.1.11.
Reference
-
1. Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer. 2005; 103:1330–1335.2. Brignardello E, Gallo M, Baldi I, Palestini N, Piovesan A, Grossi E, et al. Anaplastic thyroid carcinoma: clinical outcome of 30 consecutive patients referred to a single institution in the past 5 years. Eur J Endocrinol. 2007; 156:425–430.3. Lo TE, Jimeno CA, Paz-Pacheco E. Anaplastic thyroid cancer: experience of the Philippine General Hospital. Endocrinol Metab (Seoul). 2015; 30:195–200.4. Giuffrida D, Gharib H. Anaplastic thyroid carcinoma: current diagnosis and treatment. Ann Oncol. 2000; 11:1083–1089.5. Nagaiah G, Hossain A, Mooney CJ, Parmentier J, Remick SC. Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J Oncol. 2011; 2011:542358.6. McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001; 130:1028–1034.7. Nel CJ, van Heerden JA, Goellner JR, Gharib H, McConahey WM, Taylor WF, et al. Anaplastic carcinoma of the thyroid: a clinicopathologic study of 82 cases. Mayo Clin Proc. 1985; 60:51–58.8. Carcangiu ML, Steeper T, Zampi G, Rosai J. Anaplastic thyroid carcinoma. A study of 70 cases. Am J Clin Pathol. 1985; 83:135–158.9. Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990; 66:321–330.10. Veness MJ, Porter GS, Morgan GJ. Anaplastic thyroid carcinoma: dismal outcome despite current treatment approach. ANZ J Surg. 2004; 74:559–562.11. Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008; 66:1–9.12. Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009; 86:1065–1073.13. Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006; 66:605–612.14. Zlotnik A. Chemokines and cancer. Int J Cancer. 2006; 119:2026–2029.15. Kim CH, Broxmeyer HE. SLC/exodus2/6Ckine/TCA4 induces chemotaxis of hematopoietic progenitor cells: differential activity of ligands of CCR7, CXCR3, or CXCR4 in chemotaxis vs. suppression of progenitor proliferation. J Leukoc Biol. 1999; 66:455–461.16. Aiuti A, Tavian M, Cipponi A, Ficara F, Zappone E, Hoxie J, et al. Expression of CXCR4, the receptor for stromal cell-derived factor-1 on fetal and adult human lympho-hematopoietic progenitors. Eur J Immunol. 1999; 29:1823–1831.17. Cho SW, Kim YA, Sun HJ, Ahn HY, Lee EK, Yi KH, et al. Therapeutic potential of Dickkopf-1 in wild-type BRAF papillary thyroid cancer via regulation of β-catenin/E-cadherin signaling. J Clin Endocrinol Metab. 2014; 99:E1641–E1649.18. Tuominen VJ, Ruotoistenmaki S, Viitanen A, Jumppanen M, Isola J. ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res. 2010; 12:R56.19. Campbell MJ, Tonlaar NY, Garwood ER, Huo D, Moore DH, Khramtsov AI, et al. Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Res Treat. 2011; 128:703–711.20. Kim S, Cho SW, Min HS, Kim KM, Yeom GJ, Kim EY, et al. The expression of tumor-associated macrophages in papillary thyroid carcinoma. Endocrinol Metab (Seoul). 2013; 28:192–198.21. Sato S, Hanibuchi M, Kuramoto T, Yamamori N, Goto H, Ogawa H, et al. Macrophage stimulating protein promotes liver metastases of small cell lung cancer cells by affecting the organ microenvironment. Clin Exp Metastasis. 2013; 30:333–344.22. Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012; 7:e50946.23. Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. 2008; 15:1069–1074.24. Qing W, Fang WY, Ye L, Shen LY, Zhang XF, Fei XC, et al. Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid. 2012; 22:905–910.25. Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006; 13:453–464.26. Jung KY, Cho SW, Kim YA, Kim D, Oh BC, Park DJ, et al. Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. J Pathol Transl Med. 2015; 49:318–324.27. Piaggio F, Kondylis V, Pastorino F, Di Paolo D, Perri P, Cossu I, et al. A novel liposomal clodronate depletes tumor-associated macrophages in primary and metastatic melanoma: anti-angiogenic and anti-tumor effects. J Control Release. 2016; 223:165–177.28. Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006; 95:272–281.29. Rogers TL, Holen I. Tumour macrophages as potential targets of bisphosphonates. J Transl Med. 2011; 9:177.30. Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Hegmans JP, et al. Zoledronic acid impairs myeloid differentiation to tumour-associated macrophages in mesothelioma. Br J Cancer. 2010; 103:629–641.31. Conway JG, McDonald B, Parham J, Keith B, Rusnak DW, Shaw E, et al. Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. Proc Natl Acad Sci U S A. 2005; 102:16078–16083.32. Vishvakarma NK, Singh SM. Immunopotentiating effect of proton pump inhibitor pantoprazole in a lymphoma-bearing murine host: implication in antitumor activation of tumor-associated macrophages. Immunol Lett. 2010; 134:83–92.33. Watkins SK, Egilmez NK, Suttles J, Stout RD. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol. 2007; 178:1357–1362.34. Passaro C, Borriello F, Vastolo V, Di Somma S, Scamardella E, Gigantino V, et al. The oncolytic virus dl922-947 reduces IL-8/CXCL8 and MCP-1/CCL2 expression and impairs angiogenesis and macrophage infiltration in anaplastic thyroid carcinoma. Oncotarget. 2016; 7:1500–1515.35. Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TR, Reedquist KA, et al. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012; 375:196–206.36. Boimel PJ, Smirnova T, Zhou ZN, Wyckoff J, Park H, Coniglio SJ, et al. Contribution of CXCL12 secretion to invasion of breast cancer cells. Breast Cancer Res. 2012; 14:R23.37. Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, et al. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005; 97:1142–1151.38. Schramm B, Penn ML, Speck RF, Chan SY, De Clercq E, Schols D, et al. Viral entry through CXCR4 is a pathogenic factor and therapeutic target in human immunodeficiency virus type 1 disease. J Virol. 2000; 74:184–192.39. Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999; 283:845–848.40. Mohle R, Schittenhelm M, Failenschmid C, Bautz F, Kratz-Albers K, Serve H, et al. Functional response of leukaemic blasts to stromal cell-derived factor-1 correlates with preferential expression of the chemokine receptor CXCR4 in acute myelomonocytic and lymphoblastic leukaemia. Br J Haematol. 2000; 110:563–572.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cancers with Higher Density of Tumor-Associated Macrophages Were Associated with Poor Survival Rates
- CXC and CC Chemokine Expression by Intestinal Epithelial Cells in Response to Clostridium difficile Toxin A
- Immunohistochemical Expression of CXC Chemokine Receptor 4 and Galectin-3 in Follicular Tumors of Thyroid
- Isoorientin Suppresses Invasion of Breast and Colon Cancer Cells by Inhibition of CXC Chemokine Receptor 4 Expression
- Prognostic Implications of the Expression of CXCL16 in Breast Carcinoma