Yonsei Med J.
2015 Jan;56(1):16-23. 10.3349/ymj.2015.56.1.16.
Anthocyanin Induces Apoptosis of DU-145 Cells In Vitro and Inhibits Xenograft Growth of Prostate Cancer
- Affiliations
-
- 1Department of Urology, College of Medicine, The Catholic University of Korea, Seoul, Korea. ksw1227@catholic.ac.kr
- 2Catholic Integrative Medicine Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Korea Bio Medical Science Institute, Seoul, Korea.
- 4Department of Urology, Second Hospital of Lanzhou University, Lanzhou, China.
- KMID: 2352784
- DOI: http://doi.org/10.3349/ymj.2015.56.1.16
Abstract
- PURPOSE
To investigate the effects of anthocyanins extracted from black soybean, which have antioxidant activity, on apoptosis in vitro (in hormone refractory prostate cancer cells) and on tumor growth in vivo (in athymic nude mouse xenograft model).
MATERIALS AND METHODS
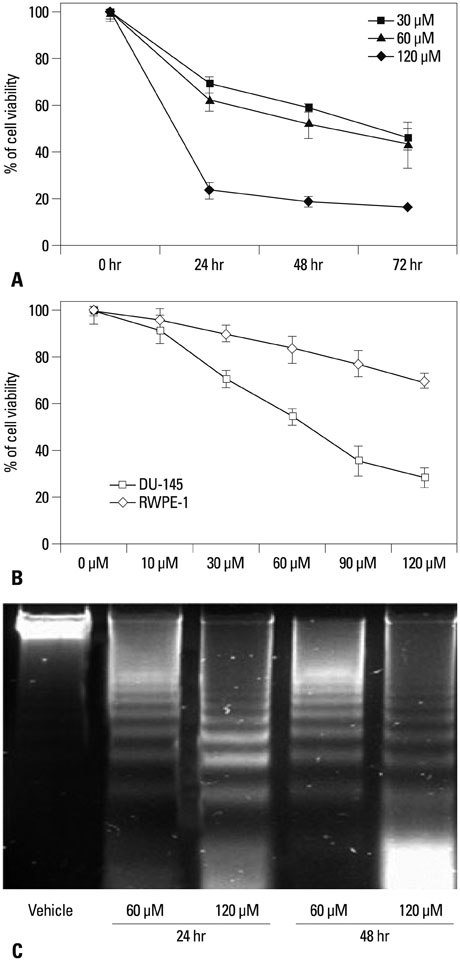
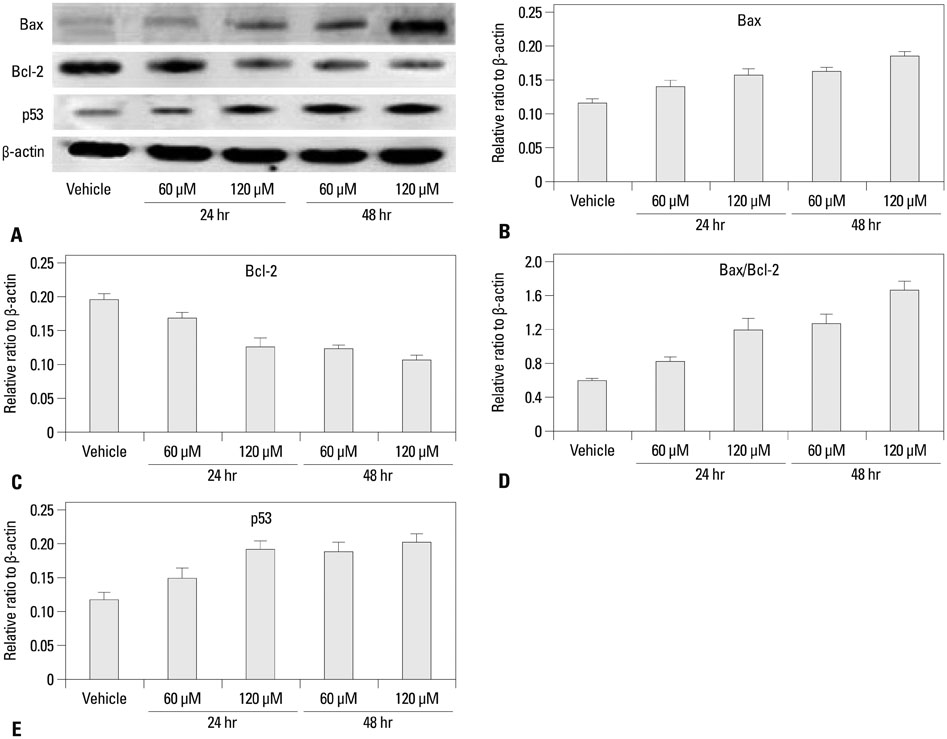
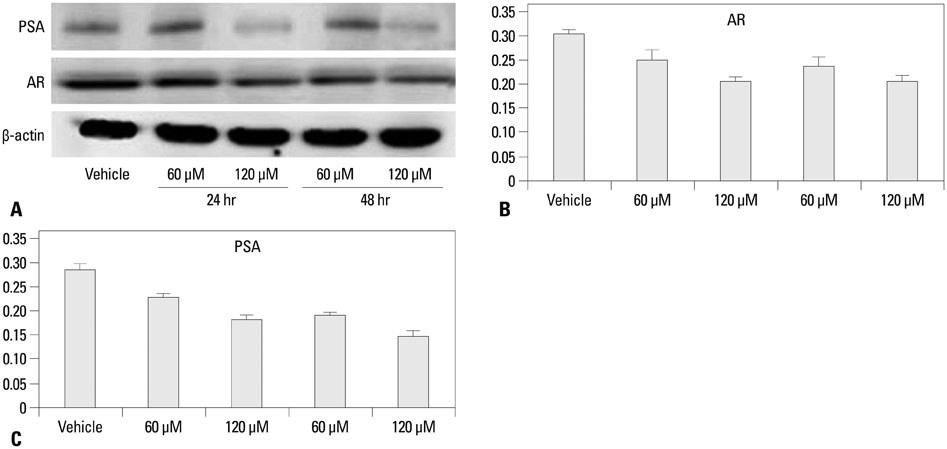
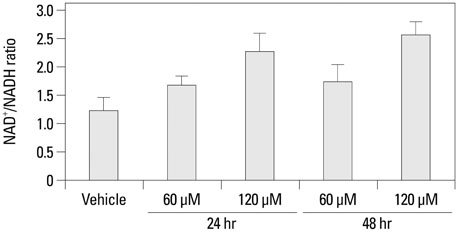
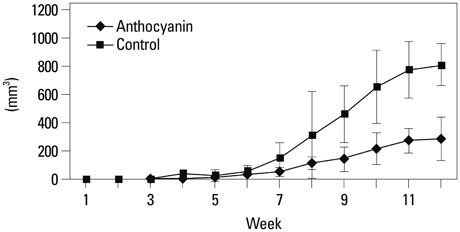
The growth and viability of DU-145 cells treated with anthocyanins were assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and apoptosis was assessed by DNA laddering. Immunoblotting was conducted to evaluate differences in the expressions of p53, Bax, Bcl, androgen receptor (AR), and prostate specific antigen (PSA). To study the inhibitory effects of anthocyanins on tumor growth in vivo, DU-145 tumor xenografts were established in athymic nude mice. The anthocyanin group was treated with daily oral anthocyanin (8 mg/kg) for 14 weeks. After 2 weeks of treatment, DU-145 cells (2x106) were inoculated subcutaneously into the right flank to establish tumor xenografts. Tumor dimensions were measured twice a week using calipers and volumes were calculated.
RESULTS
Anthocyanin treatment of DU-145 cells resulted in 1) significant increase in apoptosis in a dose-dependent manner, 2) significant decrease in p53 and Bcl-2 expressions (with increased Bax expression), and 3) significant decrease in PSA and AR expressions. In the xenograft model, anthocyanin treatment significantly inhibit tumor growth.
CONCLUSION
This study suggests that anthocyanins from black soybean inhibit the progression of prostate cancer in vitro and in a xenograft model.
Keyword
MeSH Terms
-
Animals
Anthocyanins/*pharmacology
Apoptosis/*drug effects
Cell Line, Tumor
Cell Proliferation/drug effects
Cell Survival/drug effects
Gene Expression Regulation, Neoplastic/drug effects
Humans
Male
Mice, Inbred C57BL
Mice, Nude
NAD/metabolism
Prostate-Specific Antigen/metabolism
Prostatic Neoplasms/genetics/*pathology
Receptors, Androgen/metabolism
Tumor Suppressor Protein p53/metabolism
*Xenograft Model Antitumor Assays
bcl-2-Associated X Protein/genetics/metabolism
Anthocyanins
NAD
Prostate-Specific Antigen
Receptors, Androgen
Tumor Suppressor Protein p53
bcl-2-Associated X Protein
Figure
Reference
-
1. Grönberg H. Prostate cancer epidemiology. Lancet. 2003; 361:859–864.
Article2. Won YJ, Sung J, Jung KW, Kong HJ, Park S, Shin HR, et al. Nationwide cancer incidence in Korea, 2003-2005. Cancer Res Treat. 2009; 41:122–131.3. Von Löw EC, Perabo FG, Siener R, Müller SC. Review. Facts and fiction of phytotherapy for prostate cancer: a critical assessment of preclinical and clinical data. In Vivo. 2007; 21:189–204.4. Park SK, Sakoda LC, Kang D, Chokkalingam AP, Lee E, Shin HR, et al. Rising prostate cancer rates in South Korea. Prostate. 2006; 66:1285–1291.
Article5. Demark-Wahnefried W, Robertson CN, Walther PJ, Polascik TJ, Paulson DF, Vollmer RT. Pilot study to explore effects of low-fat, flaxseed-supplemented diet on proliferation of benign prostatic epithelium and prostate-specific antigen. Urology. 2004; 63:900–904.
Article6. Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003; 349:366–381.
Article7. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006; 160:1–40.
Article8. Bostwick DG, Alexander EE, Singh R, Shan A, Qian J, Santella RM, et al. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer. 2000; 89:123–134.
Article9. Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996; 273:59–63.
Article10. Pamplona R, Barja G. Mitochondrial oxidative stress, aging and caloric restriction: the protein and methionine connection. Biochim Biophys Acta. 2006; 1757:496–508.
Article11. Warner HR. Superoxide dismutase, aging, and degenerative disease. Free Radic Biol Med. 1994; 17:249–258.12. Mo JQ, Hom DG, Andersen JK. Decreases in protective enzymes correlates with increased oxidative damage in the aging mouse brain. Mech Ageing Dev. 1995; 81:73–82.13. Malins DC, Johnson PM, Wheeler TM, Barker EA, Polissar NL, Vinson MA. Age-related radical-induced DNA damage is linked to prostate cancer. Cancer Res. 2001; 61:6025–6028.14. Malins DC, Johnson PM, Barker EA, Polissar NL, Wheeler TM, Anderson KM. Cancer-related changes in prostate DNA as men age and early identification of metastasis in primary prostate tumors. Proc Natl Acad Sci U S A. 2003; 100:5401–5406.
Article15. Jang H, Kim SJ, Yuk SM, Han DS, Ha US, Hong SH, et al. Effects of anthocyanin extracted from black soybean seed coat on spermatogenesis in a rat varicocele-induced model. Reprod Fertil Dev. 2012; 24:649–655.
Article16. Jang H, Ha US, Kim SJ, Yoon BI, Han DS, Yuk SM, et al. Anthocyanin extracted from black soybean reduces prostate weight and promotes apoptosis in the prostatic hyperplasia-induced rat model. J Agric Food Chem. 2010; 58:12686–12691.17. Gong J, Traganos F, Darzynkiewicz Z. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal Biochem. 1994; 218:314–319.18. Alessandri G, Filippeschi S, Sinibaldi P, Mornet F, Passera P, Spreafico F, et al. Influence of gangliosides on primary and metastatic neoplastic growth in human and murine cells. Cancer Res. 1987; 47:4243–4247.19. Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999; 59:7 Suppl. 1693s–1700s.
Article20. Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997; 326(Pt 1):1–16.
Article21. Akao Y, Otsuki Y, Kataoka S, Ito Y, Tsujimoto Y. Multiple subcellular localization of bcl-2: detection in nuclear outer membrane, endoplasmic reticulum membrane, and mitochondrial membranes. Cancer Res. 1994; 54:2468–2471.22. Danial NN. BCL-2 family proteins: critical checkpoints of apoptotic cell death. Clin Cancer Res. 2007; 13:7254–7263.
Article23. Ohigashi T, Ueno M, Nonaka S, Nakanoma T, Furukawa Y, Deguchi N, et al. Tyrosine kinase inhibitors reduce bcl-2 expression and induce apoptosis in androgen-dependent cells. Am J Physiol Cell Physiol. 2000; 278:C66–C72.24. Rosser CJ, Reyes AO, Vakar-Lopez F, Levy LB, Kuban DA, Hoover DC, et al. Bcl-2 is significantly overexpressed in localized radio-recurrent prostate carcinoma, compared with localized radio-naive prostate carcinoma. Int J Radiat Oncol Biol Phys. 2003; 56:1–6.
Article25. Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993; 74:609–619.
Article26. Yang E, Korsmeyer SJ. Molecular thanatopsis: a discourse on the BCL2 family and cell death. Blood. 1996; 88:386–401.
Article27. Marcelli M, Marani M, Li X, Sturgis L, Haidacher SJ, Trial JA, et al. Heterogeneous apoptotic responses of prostate cancer cell lines identify an association between sensitivity to staurosporine-induced apoptosis, expression of Bcl-2 family members, and caspase activation. Prostate. 2000; 42:260–273.
Article28. Lowe SL, Rubinchik S, Honda T, McDonnell TJ, Dong JY, Norris JS. Prostate-specific expression of Bax delivered by an adenoviral vector induces apoptosis in LNCaP prostate cancer cells. Gene Ther. 2001; 8:1363–1371.
Article29. Chang HK, Shin MS, Yang HY, Lee JW, Kim YS, Lee MH, et al. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol Pharm Bull. 2006; 29:1597–1602.
Article30. Li X, Marani M, Yu J, Nan B, Roth JA, Kagawa S, et al. Adenovirus-mediated Bax overexpression for the induction of therapeutic apoptosis in prostate cancer. Cancer Res. 2001; 61:186–191.31. Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995; 80:293–299.
Article32. Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, et al. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994; 9:1799–1805.33. Reddivari L, Vanamala J, Chintharlapalli S, Safe SH, Miller JC Jr. Anthocyanin fraction from potato extracts is cytotoxic to prostate cancer cells through activation of caspase-dependent and caspase-independent pathways. Carcinogenesis. 2007; 28:2227–2235.
Article34. Karna P, Gundala SR, Gupta MV, Shamsi SA, Pace RD, Yates C, et al. Polyphenol-rich sweet potato greens extract inhibits proliferation and induces apoptosis in prostate cancer cells in vitro and in vivo. Carcinogenesis. 2011; 32:1872–1880.
Article35. Linja MJ, Savinainen KJ, Saramäki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001; 61:3550–3555.36. Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010; 120:2715–2730.
Article37. Tilley WD, Buchanan G, Hickey TE, Bentel JM. Mutations in the androgen receptor gene are associated with progression of human prostate cancer to androgen independence. Clin Cancer Res. 1996; 2:277–285.38. Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009; 15:4792–4798.
Article39. Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004; 25:276–308.
Article40. Singh P, Uzgare A, Litvinov I, Denmeade SR, Isaacs JT. Combinatorial androgen receptor targeted therapy for prostate cancer. Endocr Relat Cancer. 2006; 13:653–666.
Article41. Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res. 2006; 12:1665–1671.
Article42. Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009; 324:787–790.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apoptosis of Prostate Cancer by Bax Gene Expression
- The Effects of Adiponectin and Leptin in the Proliferation of Prostate Cancer Cells
- Study on the Apoptosis in Human Prostate and Breast Cancer Cells
- Finasteride Increases the Expression of Hemoxygenase-1 (HO-1) and NF-E2-Related Factor-2 (Nrf2) Proteins in PC-3 Cells: Implication of Finasteride-Mediated High-Grade Prostate Tumor Occurrence
- Docetaxel Enhances Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Mediated Apoptosis in Prostate Cancer Cells via Epigenetic Gene Regulation by Enhancer of Zeste Homolog 2