Yonsei Med J.
2015 Jan;56(1):1-15. 10.3349/ymj.2015.56.1.1.
Voltage Regulation of Connexin Channel Conductance
- Affiliations
-
- 1Department of Physiology, College of Medicine, Dankook University, Cheonan, Korea.
- 2Dominic P. Purpura Department of Neuroscience, Albert Einstein College of Medicine, Bronx, NY, USA. ted.bargiello@einstein.yu.edu
- KMID: 2352783
- DOI: http://doi.org/10.3349/ymj.2015.56.1.1
Abstract
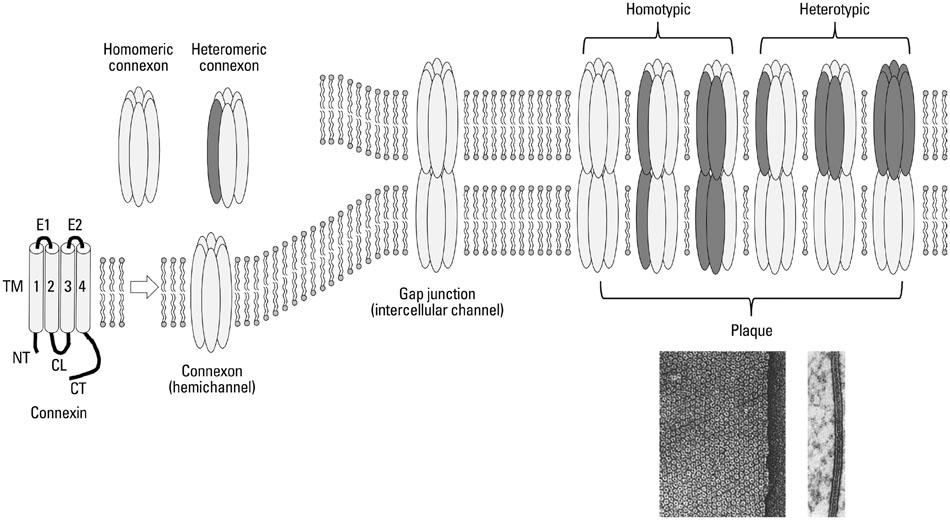
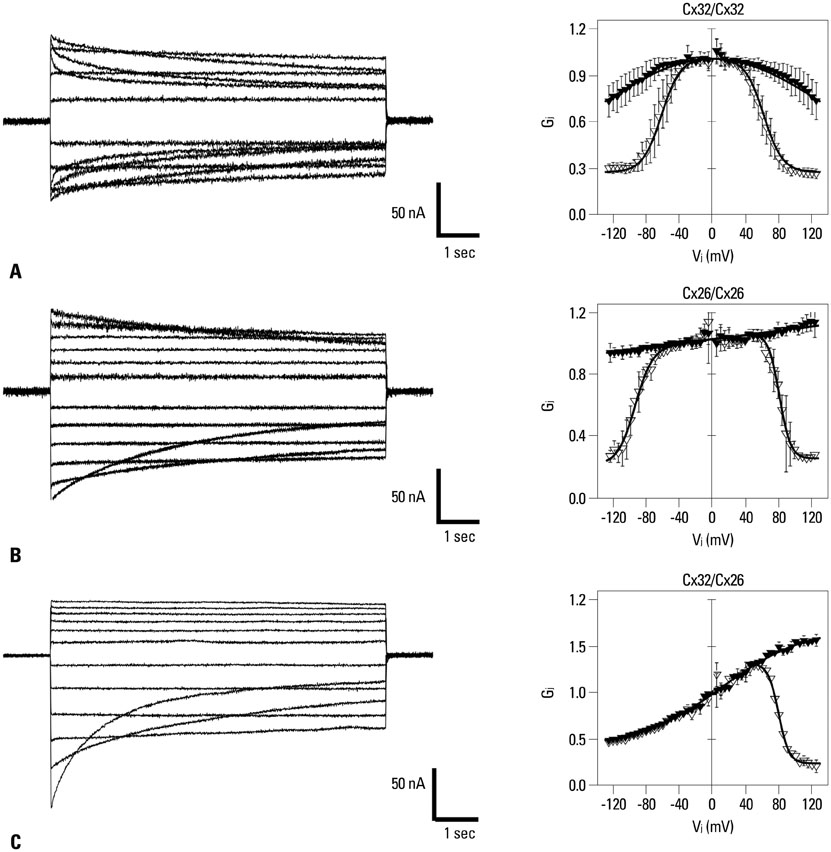
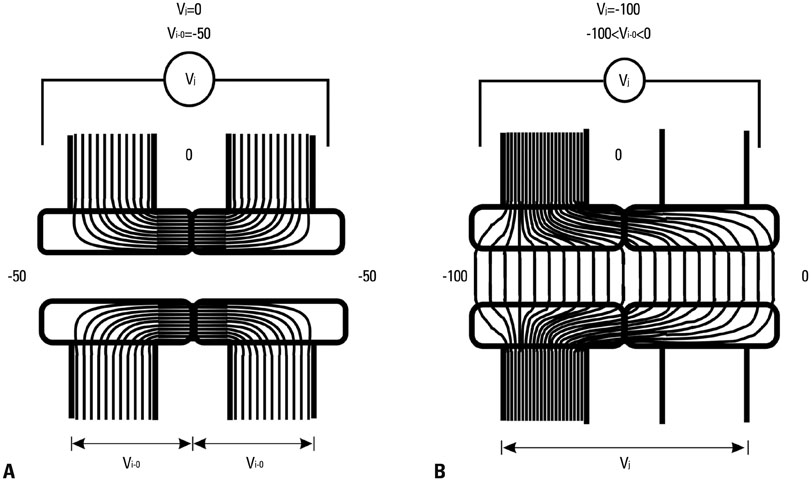
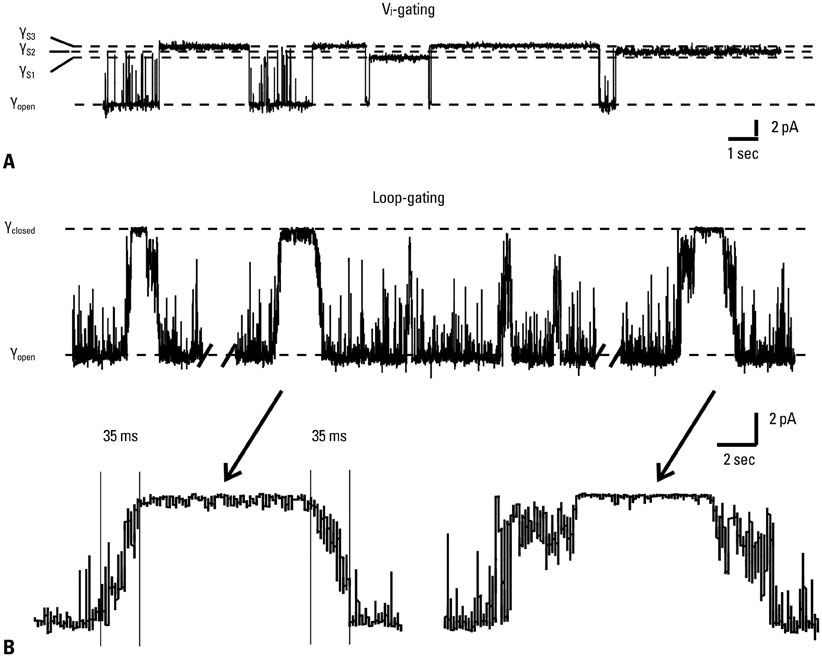
- Voltage is an important parameter that regulates the conductance of both intercellular and plasma membrane channels (undocked hemichannels) formed by the 21 members of the mammalian connexin gene family. Connexin channels display two forms of voltage-dependence, rectification of ionic currents and voltage-dependent gating. Ionic rectification results either from asymmetries in the distribution of fixed charges due to heterotypic pairing of different hemichannels, or by channel block, arising from differences in the concentrations of divalent cations on opposite sides of the junctional plaque. This rectification likely underpins the electrical rectification observed in some electrical synapses. Both intercellular and undocked hemichannels also display two distinct forms of voltage-dependent gating, termed Vj (fast)-gating and loop (slow)-gating. This review summarizes our current understanding of the molecular determinants and mechanisms underlying these conformational changes derived from experimental, molecular-genetic, structural, and computational approaches.
Keyword
MeSH Terms
Figure
Reference
-
1. Beyer EC, Berthoud VM. The Family of Connexin Genes. In : Harris AL, Locke D, editors. Connexins: a guide. New York, NY: Humana Press;2009. p. 3–26.2. Bai D, Wang AH. Extracellular domains play different roles in gap junction formation and docking compatibility. Biochem J. 2014; 458:1–10.
Article3. Phelan P, Starich TA. Innexins get into the gap. Bioessays. 2001; 23:388–396.
Article4. Bauer R, Löer B, Ostrowski K, Martini J, Weimbs A, Lechner H, et al. Intercellular communication: the Drosophila innexin multiprotein family of gap junction proteins. Chem Biol. 2005; 12:515–526.
Article5. Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochim Biophys Acta. 2013; 1828:15–22.
Article6. Siebert AP, Ma Z, Grevet JD, Demuro A, Parker I, Foskett JK. Structural and functional similarities of calcium homeostasis modulator 1 (CALHM1) ion channel with connexins, pannexins, and innexins. J Biol Chem. 2013; 288:6140–6153.
Article7. Ma Z, Siebert AP, Cheung KH, Lee RJ, Johnson B, Cohen AS, et al. Calcium homeostasis modulator 1 (CALHM1) is the pore-forming subunit of an ion channel that mediates extracellular Ca2+ regulation of neuronal excitability. Proc Natl Acad Sci U S A. 2012; 109:E1963–E1971.8. Wang N, De Bock M, Decrock E, Bol M, Gadicherla A, Vinken M, et al. Paracrine signaling through plasma membrane hemichannels. Biochim Biophys Acta. 2013; 1828:35–50.
Article9. Bukauskas FF, Jordan K, Bukauskiene A, Bennett MV, Lampe PD, Laird DW, et al. Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proc Natl Acad Sci U S A. 2000; 97:2556–2561.
Article10. Harris AL, Locke D. Permeability of connexin channels. In : Harris AL, Locke D, editors. Connexins: a guide. New York, NY: Humana Press;2009. p. 576.11. Kanaporis G, Mese G, Valiuniene L, White TW, Brink PR, Valiunas V. Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J Gen Physiol. 2008; 131:293–305.
Article12. Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, et al. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005; 568(Pt 2):459–468.
Article13. Elfgang C, Eckert R, Lichtenberg-Fraté H, Butterweck A, Traub O, Klein RA, et al. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995; 129:805–817.
Article14. Oh S, Verselis VK, Bargiello TA. Charges dispersed over the permeation pathway determine the charge selectivity and conductance of a Cx32 chimeric hemichannel. J Physiol. 2008; 586:2445–2461.
Article15. Oh S, Ri Y, Bennett MV, Trexler EB, Verselis VK, Bargiello TA. Changes in permeability caused by connexin 32 mutations underlie X-linked Charcot-Marie-Tooth disease. Neuron. 1997; 19:927–938.
Article16. Pfenniger A, Wohlwend A, Kwak BR. Mutations in connexin genes and disease. Eur J Clin Invest. 2011; 41:103–116.
Article17. Abrams CK, Freidin M. GJB1-associated X-linked Charcot-Marie-Tooth disease, a disorder affecting the central and peripheral nervous systems. Cell Tissue Res. 2014; [Epub ahead of print].
Article18. Abrams CK, Islam M, Mahmoud R, Kwon T, Bargiello TA, Freidin MM. Functional requirement for a highly conserved charged residue at position 75 in the gap junction protein connexin 32. J Biol Chem. 2013; 288:3609–3619.
Article19. Oh S, Rubin JB, Bennett MV, Verselis VK, Bargiello TA. Molecular determinants of electrical rectification of single channel conductance in gap junctions formed by connexins 26 and 32. J Gen Physiol. 1999; 114:339–364.
Article20. Bukauskas FF, Bukauskiene A, Verselis VK. Conductance and permeability of the residual state of connexin43 gap junction channels. J Gen Physiol. 2002; 119:171–185.
Article21. Furshpan EJ, Potter DD. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959; 145:289–325.
Article22. Auerbach AA, Bennett MV. A rectifying electrotonic synapse in the central nervous system of a vertebrate. J Gen Physiol. 1969; 53:211–237.
Article23. Kwon T, Harris AL, Rossi A, Bargiello TA. Molecular dynamics simulations of the Cx26 hemichannel: evaluation of structural models with Brownian dynamics. J Gen Physiol. 2011; 138:475–493.
Article24. Trexler EB, Bukauskas FF, Kronengold J, Bargiello TA, Verselis VK. The first extracellular loop domain is a major determinant of charge selectivity in connexin46 channels. Biophys J. 2000; 79:3036–3051.
Article25. Hamzei-Sichani F, Davidson KG, Yasumura T, Janssen WG, Wearne SL, Hof PR, et al. Mixed Electrical-Chemical Synapses in Adult Rat Hippocampus are Primarily Glutamatergic and Coupled by Connexin-36. Front Neuroanat. 2012; 6:13.
Article26. Bautista W, Rash JE, Vanderpool KG, Yasumura T, Nagy JI. Re-evaluation of connexins associated with motoneurons in rodent spinal cord, sexually dimorphic motor nuclei and trigeminal motor nucleus. Eur J Neurosci. 2014; 39:757–770.
Article27. Palacios-Prado N, Chapuis S, Panjkovich A, Fregeac J, Nagy JI, Bukauskas FF. Molecular determinants of magnesium-dependent synaptic plasticity at electrical synapses formed by connexin36. Nat Commun. 2014; 5:4667.
Article28. Palacios-Prado N, Hoge G, Marandykina A, Rimkute L, Chapuis S, Paulauskas N, et al. Intracellular magnesium-dependent modulation of gap junction channels formed by neuronal connexin36. J Neurosci. 2013; 33:4741–4753.
Article29. Rash JE, Curti S, Vanderpool KG, Kamasawa N, Nannapaneni S, Palacios-Prado N, et al. Molecular and functional asymmetry at a vertebrate electrical synapse. Neuron. 2013; 79:957–969.
Article30. Bargiello T, Brink P. Voltage-Gating Mechanisms of Connexin Channels. In : Harris AL, Locke D, editors. Connexins: a guide. New York, NY: Humana Press;2009. p. 103–128.31. Harris AL, Spray DC, Bennett MV. Kinetic properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981; 77:95–117.
Article32. Spray DC, Harris AL, Bennett MV. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981; 77:77–93.
Article33. Barrio LC, Suchyna T, Bargiello T, Xu LX, Roginski RS, Bennett MV, et al. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc Natl Acad Sci U S A. 1991; 88:8410–8414.
Article34. Verselis VK, Bennett MV, Bargiello TA. A voltage-dependent gap junction in Drosophila melanogaster. Biophys J. 1991; 59:114–126.
Article35. Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991; 115:1077–1089.
Article36. Trexler EB, Bennett MV, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. Proc Natl Acad Sci U S A. 1996; 93:5836–5841.
Article37. Bargiello TA, Tang Q, Oh S, Kwon T. Voltage-dependent conformational changes in connexin channels. Biochim Biophys Acta. 2012; 1818:1807–1822.
Article38. Bukauskas FF, Verselis VK. Gap junction channel gating. Biochim Biophys Acta. 2004; 1662:42–60.
Article39. Pfahnl A, Zhou XW, Werner R, Dahl G. A chimeric connexin forming gap junction hemichannels. Pflugers Arch. 1997; 433:773–779.
Article40. Bukauskas FF, Angele AB, Verselis VK, Bennett MV. Coupling asymmetry of heterotypic connexin 45/ connexin 43-EGFP gap junctions: properties of fast and slow gating mechanisms. Proc Natl Acad Sci U S A. 2002; 99:7113–7118.
Article41. Contreras JE, Sáez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci U S A. 2003; 100:11388–11393.
Article42. Oh S, Rivkin S, Tang Q, Verselis VK, Bargiello TA. Determinants of gating polarity of a connexin 32 hemichannel. Biophys J. 2004; 87:912–928.
Article43. Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature. 1994; 368:348–351.
Article44. Oh S, Abrams CK, Verselis VK, Bargiello TA. Stoichiometry of transjunctional voltage-gating polarity reversal by a negative charge substitution in the amino terminus of a connexin32 chimera. J Gen Physiol. 2000; 116:13–31.
Article45. Anumonwo JM, Taffet SM, Gu H, Chanson M, Moreno AP, Delmar M. The carboxyl terminal domain regulates the unitary conductance and voltage dependence of connexin40 gap junction channels. Circ Res. 2001; 88:666–673.
Article46. Moreno AP, Chanson M, Elenes S, Anumonwo J, Scerri I, Gu H, et al. Role of the carboxyl terminal of connexin43 in transjunctional fast voltage gating. Circ Res. 2002; 90:450–457.
Article47. Shibayama J, Gutiérrez C, González D, Kieken F, Seki A, Carrión JR, et al. Effect of charge substitutions at residue his-142 on voltage gating of connexin43 channels. Biophys J. 2006; 91:4054–4063.
Article48. Revilla A, Castro C, Barrio LC. Molecular dissection of transjunctional voltage dependence in the connexin-32 and connexin-43 junctions. Biophys J. 1999; 77:1374–1383.
Article49. Rubin JB, Verselis VK, Bennett MV, Bargiello TA. Molecular analysis of voltage dependence of heterotypic gap junctions formed by connexins 26 and 32. Biophys J. 1992; 62:183–193.
Article50. Srinivas M, Kronengold J, Bukauskas FF, Bargiello TA, Verselis VK. Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophys J. 2005; 88:1725–1739.
Article51. Peracchia C, Peracchia LL. Inversion of both gating polarity and CO2 sensitivity of voltage gating with D3N mutation of Cx50. Am J Physiol Cell Physiol. 2005; 288:C1381–C1389.52. Purnick PE, Oh S, Abrams CK, Verselis VK, Bargiello TA. Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin 32. Biophys J. 2000; 79:2403–2415.
Article53. Purnick PE, Benjamin DC, Verselis VK, Bargiello TA, Dowd TL. Structure of the amino terminus of a gap junction protein. Arch Biochem Biophys. 2000; 381:181–190.
Article54. Kalmatsky BD, Bhagan S, Tang Q, Bargiello TA, Dowd TL. Structural studies of the N-terminus of Connexin 32 using 1H NMR spectroscopy. Arch Biochem Biophys. 2009; 490:9–16.
Article55. Kalmatsky BD, Batir Y, Bargiello TA, Dowd TL. Structural studies of N-terminal mutants of connexin 32 using (1)H NMR spectroscopy. Arch Biochem Biophys. 2012; 526:1–8.
Article56. Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, et al. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature. 2009; 458:597–602.
Article57. Oshima A, Tani K, Hiroaki Y, Fujiyoshi Y, Sosinsky GE. Three-dimensional structure of a human connexin26 gap junction channel reveals a plug in the vestibule. Proc Natl Acad Sci U S A. 2007; 104:10034–10039.
Article58. Oshima A, Tani K, Hiroaki Y, Fujiyoshi Y, Sosinsky GE. Projection structure of a N-terminal deletion mutant of connexin 26 channel with decreased central pore density. Cell Commun Adhes. 2008; 15:85–93.
Article59. Oshima A, Tani K, Toloue MM, Hiroaki Y, Smock A, Inukai S, et al. Asymmetric configurations and N-terminal rearrangements in connexin26 gap junction channels. J Mol Biol. 2011; 405:724–735.
Article60. Cha A, Snyder GE, Selvin PR, Bezanilla F. Atomic scale movement of the voltage-sensing region in a potassium channel measured via spectroscopy. Nature. 1999; 402:809–813.
Article61. Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol Rev. 2000; 80:555–592.
Article62. Locke D, Bian S, Li H, Harris AL. Post-translational modifications of connexin26 revealed by mass spectrometry. Biochem J. 2009; 424:385–398.
Article63. Ek-Vitorín JF, Calero G, Morley GE, Coombs W, Taffet SM, Delmar M. PH regulation of connexin43: molecular analysis of the gating particle. Biophys J. 1996; 71:1273–1284.
Article64. Kwon T, Dowd TL, Bargiello TA. The carboxyl terminal residues 220-283 are not required for voltage gating of a chimeric connexin32 hemichannel. Biophys J. 2013; 105:1376–1382.
Article65. Verselis VK, Srinivas M. Divalent cations regulate connexin hemichannels by modulating intrinsic voltage-dependent gating. J Gen Physiol. 2008; 132:315–327.
Article66. Kwon T, Tang Q, Bargiello TA. Voltage-dependent gating of the Cx32*43E1 hemichannel: conformational changes at the channel entrances. J Gen Physiol. 2013; 141:243–259.
Article67. Lopez W, Gonzalez J, Liu Y, Harris AL, Contreras JE. Insights on the mechanisms of Ca(2+) regulation of connexin26 hemichannels revealed by human pathogenic mutations (D50N/Y). J Gen Physiol. 2013; 142:23–35.
Article68. Lopez W, Liu Y, Harris AL, Contreras JE. Divalent regulation and intersubunit interactions of human connexin26 (Cx26) hemichannels. Channels (Austin). 2014; 8:1–4.
Article69. Gómez-Hernández JM, de Miguel M, Larrosa B, González D, Barrio LC. Molecular basis of calcium regulation in connexin-32 hemichannels. Proc Natl Acad Sci U S A. 2003; 100:16030–16035.
Article70. Müller DJ, Hand GM, Engel A, Sosinsky GE. Conformational changes in surface structures of isolated connexin 26 gap junctions. EMBO J. 2002; 21:3598–3607.
Article71. Harris AL, Contreras JE. Motifs in the permeation pathway of connexin channels mediate voltage and Ca (2+) sensing. Front Physiol. 2014; 5:113.72. Vanden-Eijnden E. Transition path theory. Lect Notes Phys. 2006; 703:453–493.
Article73. Weinan E, Vanden-Eijnden E. Towards a theory of transition paths. J Stat Phys. 2006; 123:503–523.
Article74. Tang Q, Dowd TL, Verselis VK, Bargiello TA. Conformational changes in a pore-forming region underlie voltage-dependent "loop gating" of an unapposed connexin hemichannel. J Gen Physiol. 2009; 133:555–570.
Article75. Verselis VK, Trelles MP, Rubinos C, Bargiello TA, Srinivas M. Loop gating of connexin hemichannels involves movement of pore-lining residues in the first extracellular loop domain. J Biol Chem. 2009; 284:4484–4493.
Article76. Holmgren M, Shin KS, Yellen G. The activation gate of a voltage-gated K+ channel can be trapped in the open state by an intersubunit metal bridge. Neuron. 1998; 21:617–621.
Article77. Nightingale ER Jr. Phenomenological theory of ion solvation. effective radii of hydrated ions. J Phys Chem. 1959; 63:1381–1387.
Article78. Kwon T, Roux B, Jo S, Klauda JB, Harris AL, Bargiello TA. Molecular dynamics simulations of the Cx26 hemichannel: insights into voltage-dependent loop-gating. Biophys J. 2012; 102:1341–1351.
Article79. Zonta F, Polles G, Zanotti G, Mammano F. Permeation pathway of homomeric connexin 26 and connexin 30 channels investigated by molecular dynamics. J Biomol Struct Dyn. 2012; 29:985–998.
Article80. Araya-Secchi R, Perez-Acle T, Kang SG, Huynh T, Bernardin A, Escalona Y, et al. Characterization of a novel water pocket inside the human Cx26 hemichannel structure. Biophys J. 2014; 107:599–612.
Article81. Bennett B, Purdy M, Baker K, McIntire W, Stevens R, Zhang Q, et al. X-ray structure of the Cx26 gap junction channel and comparison with the Cryo-EM structure of Cx43. Biophys J. 2013; 104:Suppl 1. 42a–43a.
Article82. Im W, Seefeld S, Roux B. A Grand Canonical Monte Carlo-Brownian dynamics algorithm for simulating ion channels. Biophys J. 2000; 79:788–801.
Article83. Noskov SY, Im W, Roux B. Ion permeation through the alpha-hemolysin channel: theoretical studies based on Brownian dynamics and Poisson-Nernst-Plank electrodiffusion theory. Biophys J. 2004; 87:2299–2309.
Article84. Roux B, Allen T, Bernèche S, Im W. Theoretical and computational models of biological ion channels. Q Rev Biophys. 2004; 37:15–103.
Article85. Lee KI, Rui H, Pastor RW, Im W. Brownian dynamics simulations of ion transport through the VDAC. Biophys J. 2011; 100:611–619.
Article86. Khalili-Araghi F, Jogini V, Yarov-Yarovoy V, Tajkhorshid E, Roux B, Schulten K. Calculation of the gating charge for the Kv1.2 voltage-activated potassium channel. Biophys J. 2010; 98:2189–2198.
Article87. Pan AC, Cuello LG, Perozo E, Roux B. Thermodynamic coupling between activation and inactivation gating in potassium channels revealed by free energy molecular dynamics simulations. J Gen Physiol. 2011; 138:571–580.
Article88. Vargas E, Yarov-Yarovoy V, Khalili-Araghi F, Catterall WA, Klein ML, Tarek M, et al. An emerging consensus on voltage-dependent gating from computational modeling and molecular dynamics simulations. J Gen Physiol. 2012; 140:587–594.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The properties of voltage-sensitive chloride channels
- Properties of stretch-activated K+ channels in an G292 osteoblast-like cell
- The role of intracellular Mg2+ in regulation of Ca2+-activated K+ channel in pulmonary arterial smooth muscle cells of the rabbit
- Altered Expressions of Calcium-Activated Potassium Channel and Connexin in Bladder Mucosae of Stress Urinary Incontinence Patients with Overactive Bladder Symptoms
- Electrophysiological Properties of Ion Channels in Ascaris suum Tissue Incorporated into Planar Lipid Bilayers