Korean J Urol.
2006 Jul;47(7):722-728. 10.4111/kju.2006.47.7.722.
Altered Expressions of Calcium-Activated Potassium Channel and Connexin in Bladder Mucosae of Stress Urinary Incontinence Patients with Overactive Bladder Symptoms
- Affiliations
-
- 1Department of Urology, Catholic University of Daegu School of Medicine, Daegu, Korea. jeongkl@kumc.or.kr
- 2Department of Physiology, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 2294312
- DOI: http://doi.org/10.4111/kju.2006.47.7.722
Abstract
-
PURPOSE: The aim of this study was to examine how the mRNA and protein levels of calcium activated Kchannel (K(Ca)) and connexin (Cx) change in association with overactive bladder in the bladder mucosae of stress urinary incontinence (SUI) patients.
MATERIALS AND METHODS
Twenty SUI patients were included in our study. Bladder mucosae were obtained, with using cold cup biopsy forceps, from the patients suffering with genuine stress urinary incontinence (group 1, n=7), from the patients suffering with SUI along with urgency and frequency (group 2, n=6), and from the patients suffering with mixed incontinence (group 3, n=7).
RESULTS
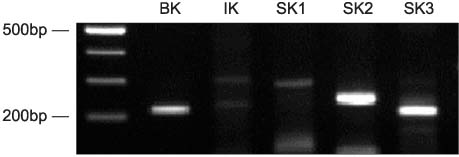
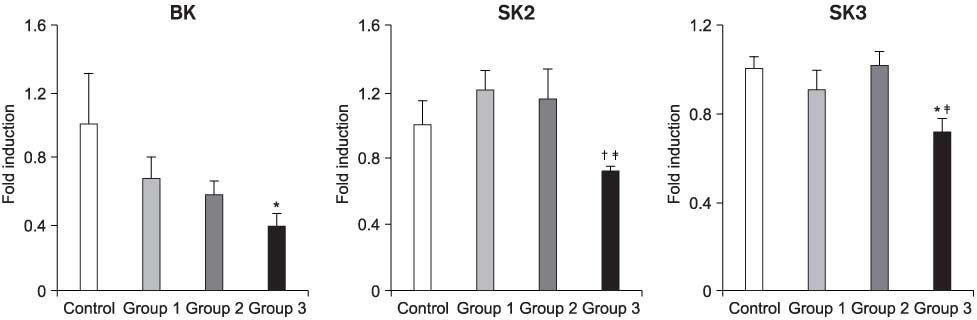
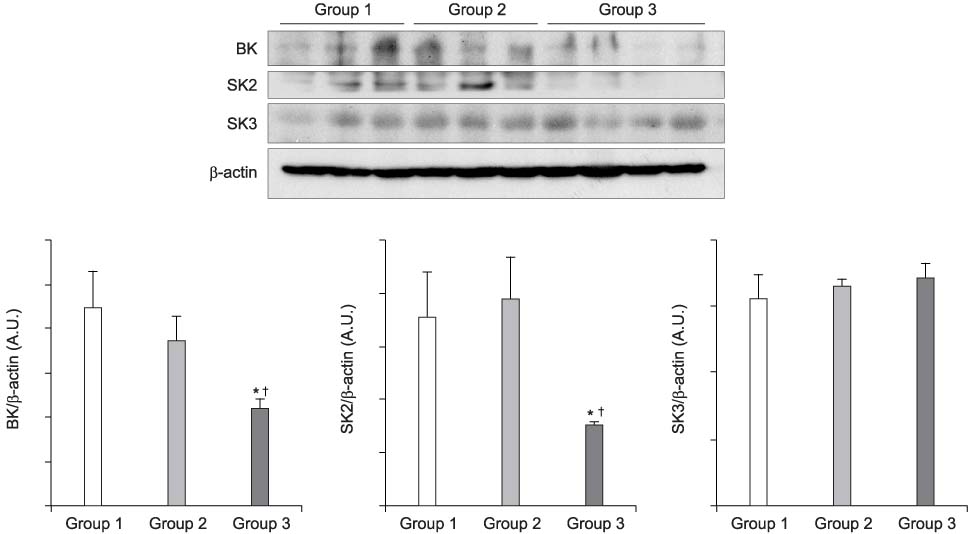
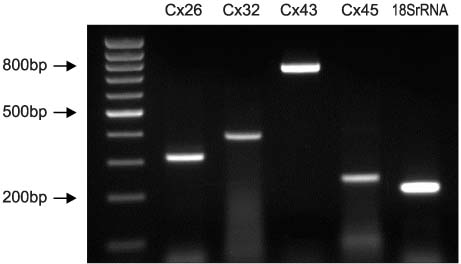
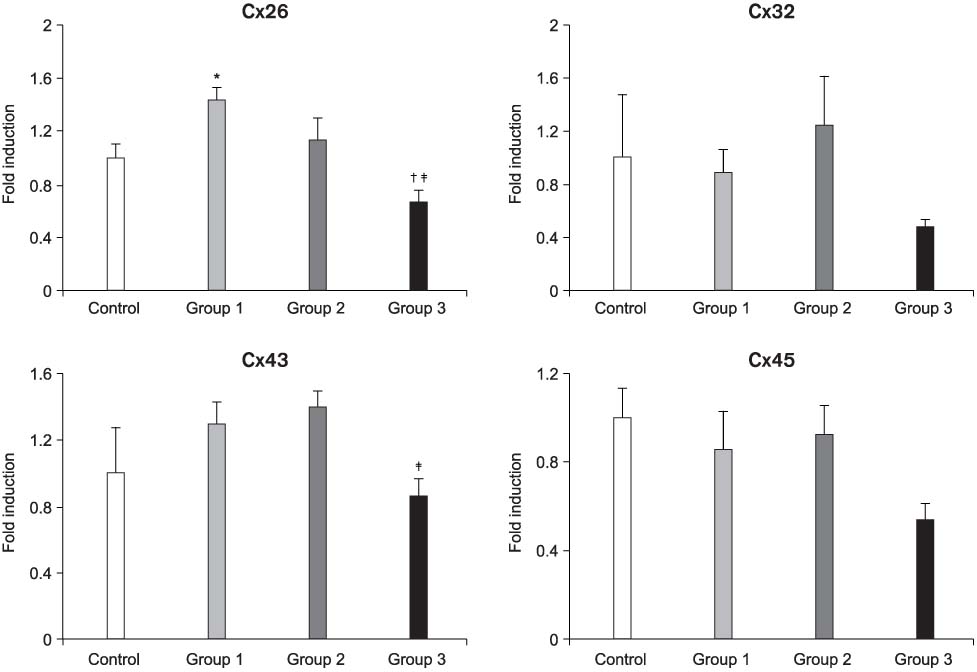
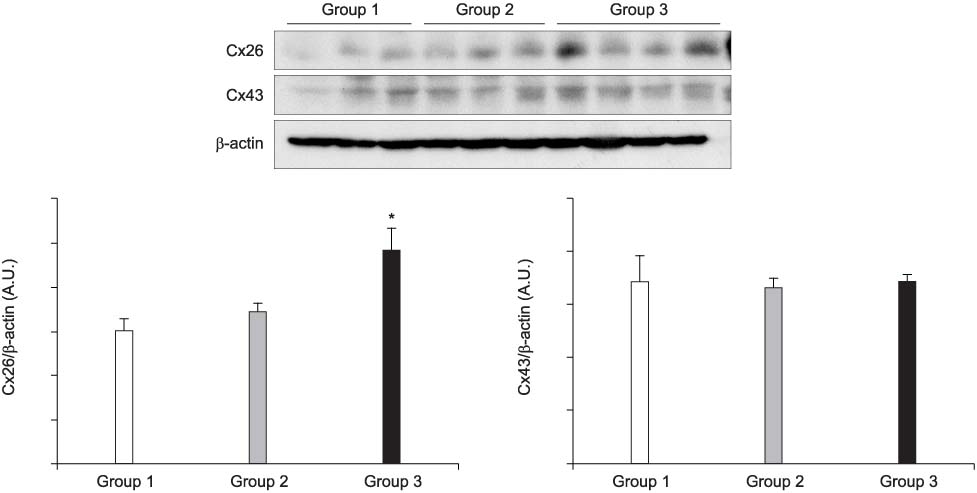
The mRNA transcripts of type 2 (SK2) and type 3 (SK3) small conductance K(Ca), Cx26, and Cx43 were highly expressed in the bladder mucosa. The message of large conductance K(Ca)(BK) was significantly decreased in group 3 compared with that in the controls. The SK2 and Cx26 messages in group 3 were also lower than those in groups 1 and 2. In the presence of urge incontinence, the BK and SK2 protein levels were decreased and the Cx26 protein expression was significantly increased in the bladder mucosa of the SUI patients. In contrast, there were no significant differences in the mRNA and protein levels of K(Ca)s and Cxs between groups 1 and group 2.
CONCLUSIONS
Downregulation of both BK and SK2 and upregulation of Cx26 in the bladder mucosa of MI patients may contribute to the alterations of urothelial instability, and this correlate with the symptom severity of bladder instability in SUI patients.
Keyword
MeSH Terms
-
Biopsy
Calcium
Connexin 43
Connexins
Down-Regulation
Humans
Mucous Membrane*
Potassium Channels, Calcium-Activated*
RNA, Messenger
Surgical Instruments
Up-Regulation
Urinary Bladder*
Urinary Bladder, Overactive*
Urinary Incontinence*
Urinary Incontinence, Stress
Urinary Incontinence, Urge
Urothelium
Calcium
Connexin 43
Connexins
Potassium Channels, Calcium-Activated
RNA, Messenger
Figure
Reference
-
1. Badawi JK, Langbein S. Current diagnostics and therapy of the overactive bladder and urge incontinence. Dtsch Med Wochenschr. 2005. 130:1503–1506.2. Kumar V, Cross RL, Chess-Williams R, Chapple CR. Recent advances in basic science for overactive bladder. Curr Opin Urol. 2005. 15:222–226.3. Herbison P, Hay-Smith J, Ellis G, Moore K. Effectiveness of the anticholinergic drugs compared with placebo in the treatment of overactive bladder: systemic review. BMJ. 2003. 326:841–844.4. Chess-Williams R. Potential therapeutic targets for the treatment of detrusor overactivity. Expert Opin Ther Targets. 2004. 8:95–106.5. Araki I, Du S, Kamiyama M, Mikami Y, Matsushita K, Komuro M, et al. Overexpression of epithelial sodium channels in epithelium of human urinary bladder with outlet obstruction. Urology. 2004. 64:1255–1260.6. de Groat WC. The urothelium in overactive bladder: passive bystander or active participant? Urology. 2004. 64:6 Suppl 1. 7–11.7. Levin RM, Monson FC, Haugaard N, Buttyan R, Hudson A, Roelofs M, et al. Genetic and cellular characteristics of bladder outlet obstruction. Urol Clin North Am. 1995. 22:263–283.8. Malkowicz SB, Wein AJ, Elbadawi A, Van Arsdalen K, Ruggieri MR, Levin RM. Acute biochemical and functional alterations in the partially obstructed rabbit urinary bladder. J Urol. 1986. 136:1324–1329.9. Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol. 2005. 289:F604–F610.10. Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004. 279:36746–36752.11. Gopalakrishnan M, Shieh CC. Potassium channel subtypes as molecular targets for overactive bladder and other urological disorders. Expert Opin Ther Targets. 2004. 8:437–458.12. Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, et al. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol. 2003. 551:893–903.13. Karicheti V, Christ GJ. Physiological roles for K+ channels and gap junctions in urogenital smooth muscle: implications for improved understanding of urogenital function, disease and therapy. Curr Drug Targets. 2001. 2:1–20.14. van Baren MJ, Heutink P. The PCR suite. Bioinformatics. 2004. 20:591–593.15. Ashcroft FM. Ion channels and disease. 2000. San Diego: Academic Press;125–160.16. Trivedi S, Potter-Lee L, Li JH, Yasay GD, Russell K, Ohmacht CJ, et al. Calcium dependent K-channels in guinea pig and human urinary bladder. Biochem Biophys Res Commun. 1995. 213:404–409.17. Chen MX, Gorman SA, Benson B, Singh K, Hieble P, Michel MC, et al. Small and intermediate conductance Ca(2+)-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn-Schmiedebergs Arch Pharmacol. 2004. 369:602–615.18. Nakamura T, Kimura J, Yamaguchi O. Muscarinic M2 receptors inhibit Ca2+-activated K+ channels in rat bladder smooth muscle. Int J Urol. 2002. 9:689–696.19. Sui GP, Coppen SR, Dupont E, Rothery S, Gillespie J, Newgreen D, et al. Impedance measurements and connexin expression in human detrusor muscle from stable and unstable bladders. BJU Int. 2003. 92:297–305.20. Sui GP, Rothery S, Dupont E, Fry CH, Severs NJ. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int. 2002. 90:118–129.21. Kim JC, Kim DB, Seo SI, Park YH, Hwang TK. Effects of connexin expression on unstable bladder after relief of bladder outlet obstruction in rat. Korean J Urol. 2003. 44:585–591.22. Tawadros T, Meda P, Leisinger HJ, Waeber G, Haefliger JA. Connexin26 is regulated in rat urothelium by the scaffold protein IB1/JIP-1. Cell Commun Adhes. 2001. 8:303–306.23. Gee J, Tanaka M, Grossman HB. Connexin 26 is abnormally expressed in bladder cancer. J Urol. 2003. 169:1135–1137.24. Haefliger JA, Tissieres P, Tawadros T, Formenton A, Beny JL, Nicod P, et al. Connexins 43 and 26 are differentially increased after rat bladder outlet obstruction. Exp Cell Res. 2002. 274:216–225.25. Abrams P. Describing bladder storage function: overactive bladder syndrome and detrusor overactivity. Urology. 2003. 62:5 Suppl 2. 28–37.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 17Beta-Estradiol Inhibits Calcium-Activated Potassium Channel Expressions in Rat Whole Bladder
- Clinical implications of underactive bladder
- Overactive Bladder
- Socioeconomic Costs of Overactive Bladder and Stress Urinary Incontinence in Korea
- Correlation analysis about effect of cardiovascular risk factors and disease in women on overactive bladder and stress urinary incontinence