Transl Clin Pharmacol.
2016 Sep;24(3):147-151. 10.12793/tcp.2016.24.3.147.
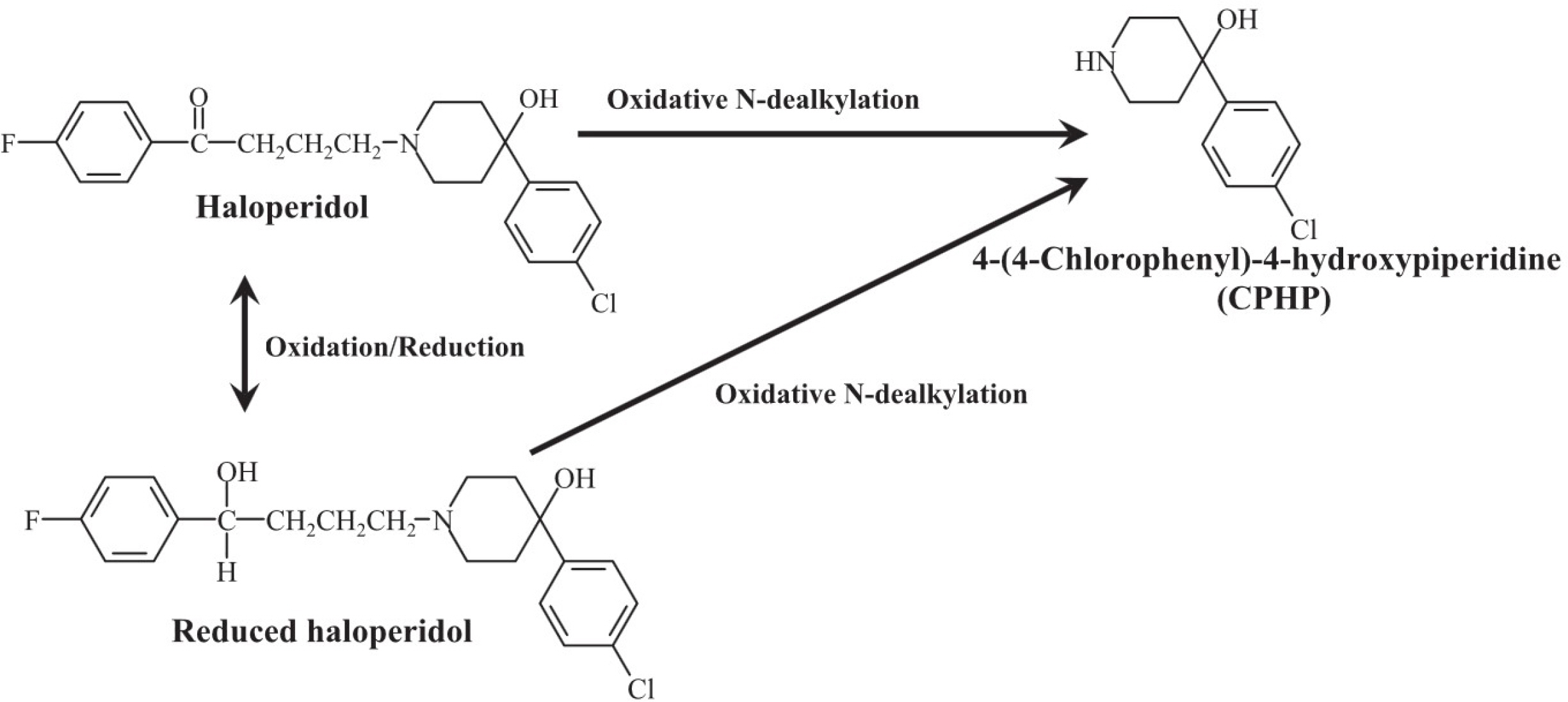
Development and validation of a HPLC-UV method for 4-(4-chlorophenyl)-4-hydroxypiperidine (CPHP), a toxic metabolite of haloperidol, in humans: providing in vivo evidence of CYP3A4-mediated CPHP formation
- Affiliations
-
- 1Department of Clinical Pharmacology and Toxicology, Anam Hospital, Korea University College of Medicine, Seoul 02841, Korea. jypark21@korea.ac.kr
- 2Department of Pharmacology and PharmacoGenomics Research Center, Inje University College of Medicine, Busan 47392, Korea.
- KMID: 2351802
- DOI: http://doi.org/10.12793/tcp.2016.24.3.147
Abstract
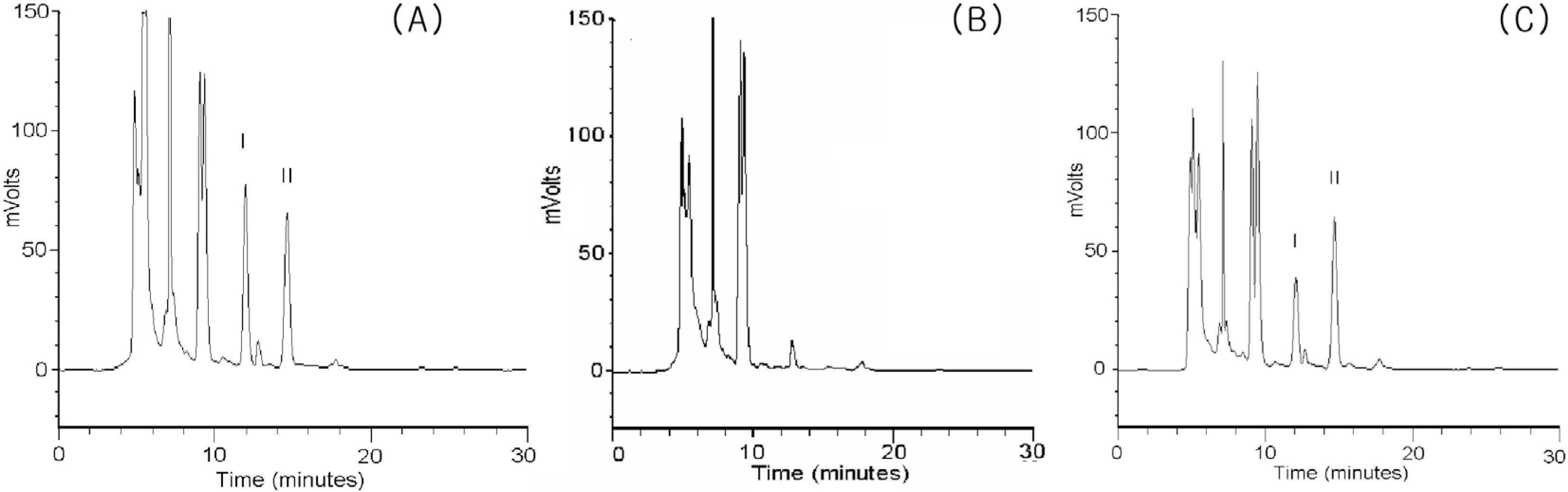
- We developed a high-performance liquid chromatographic procedure for the determination of 4-(4-chlorophenyl)-4-hydroxypiperidine (CPHP), a toxic metabolite of haloperidol, in human. Chromatographic analysis was performed on a reverse-phase Câ‚₈ column with a mobile phase containing 50 mM potassium phosphate buffer/acetonitrile (75:25, vol/vol) using UV detection with a wavelength of 220 nm. The limits of detection for CPHP were 1 ng/ml in urine and the assay was linear over the concentration range of 2-500 ng/ml for urine. This analytical method was applied to measure CPHP in human. Nineteen healthy subjects were enrolled and all subjects received a single oral dose of 5 mg haloperidol following a treatment of placebo or itraconazole at 200 mg/day for 10 days in a randomized crossover manner. CPHP was detected in urine samples and average recovered amount of CPHP was 81.31 µg/24 hr in the placebo phase and it was significantly reduced to 30.34 µg/24 hr after itraconazole treatment. The finding provides in vivo evidence that CPHP is an in vivo metabolite of haloperidol in human and its formation is mediated by CYP3A4.
Keyword
MeSH Terms
Figure
Reference
-
1.Kudo S., Ishizaki T. Pharmacokinetics of haloperidol: an update. Clin Pharmacokinet. 1999. 37:435–456.2.Usuki E., Van der Schyf CJ., Castagnoli N Jr. Metabolism of haloperidol and its tetrahydropyridine dehydration product HPTP. Drug Metab Rev. 1998. 30:809–826.
Article3.Shin JG., Kane K., Flockhart DA. Potent inhibition of CYP2D6 by haloperidol metabolites: stereoselective inhibition by reduced haloperidol. Br J Clin Pharmacol. 2001. 51:45–52.
Article4.Fang J., Yu PH., Gorrod JW., Boulton AA. Inhibition of monoamine oxidases by haloperidol and its metabolites: pharmacological implications for the chemotherapy of schizophrenia. Psychopharmacology (Berl). 1995. 118:206–212.
Article5.Rollema H., Skolnik M., D'Engelbronner J., Igarashi K., Usuki E., Castagnoli N Jr. MPP(+)-like neurotoxicity of a pyridinium metabolite derived from haloperidol: in vivo microdialysis and in vitro mitochondrial studies. J Pharmacol Exp Ther. 1994. 268:380–387.6.Wright AM., Bempong J., Kirby ML., Barlow RL., Bloomquist JR. Effects of haloperidol metabolites on neurotransmitter uptake and release: possible role in neurotoxicity and tardive dyskinesia. Brain Res. 1998. 788:215–222.
Article7.Igarashi K., Kasuya F., Fukui M., Usuki E., Castagnoli N Jr. Studies on the metabolism of haloperidol (HP): the role of CYP3A in the production of the neurotoxic pyridinium metabolite HPP+ found in rat brain following ip administration of HP. Life Sci. 1995. 57:2439–2446.
Article8.Petzer JP., Bergh JJ., Mienie LJ., Castagnoli N Jr.., Van der Schyf CJ. Metabolic defects caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and by HPTP (the tetrahydropyridinyl analog of haloperidol), in rats. Life Sci. 2000. 66:1949–1954.9.Pan LP., Wijnant P., De Vriendt C., Rosseel MT., Belpaire FM. Characterization of the cytochrome P450 isoenzymes involved in the in vitro N-dealkylation of haloperidol. Br J Clin Pharmacol. 1997. 44:557–564.10.Bowen WD., Moses EL., Tolentino PJ., Walker JM. Metabolites of haloperidol display preferential activity at sigma receptors compared to dopamine D-2 receptors. Eur J Pharmacol. 1990. 177:111–118.11.Fang J., Yu PH. Effect of haloperidol and its metabolites on dopamine and noradrenaline uptake in rat brain slices. Psychopharmacology (Berl). 1995. 121:379–384.
Article12.Fukuoka T., Nakano M., Kohda A., Okuno Y., Matsuo M. The common marmoset (Callithrix jacchus) as a model for neuroleptic-induced acute dystonia. Pharmacol Biochem Behav. 1997. 58:947–953.
Article13.Fornai F., Schlüter OM., Lenzi P., Gesi M., Ruffoli R., Ferrucci M, et al. Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc Natl Acad Sci USA. 2005. 102:3413–3418.14.Arinobu T., Hattori H., Iwai M., Ishii A., Kumazawa T., Suzuki O, et al. Liquid chromatographic-mass spectrometric determination of haloperidol and its metabolites in human plasma and urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2002. 776:107–113.
Article15.Fang J., Baker GB., Coutts RT. Determination of 4-(4-chlorophenyl)-4-hy-droxypiperidine, a metabolite of haloperidol, by gas chromatography with electron-capture detection. J Chromatogr B Biomed Appl. 1996. 682:283–288.
Article16.Higashi Y., Nakamura S., Fujii Y. Sensitive determination of 4-(4-chlorophenyl)-4-hydroxypiperidine, a metabolite of haloperidol, in a rat biological sample by HPLC with fluorescence detection after pre-column derivatization using 4-fluoro-7-nitro-2,1,3-benzoxadiazole. Biomed Chromatogr. 2006. 20:964–970.
Article17.Park JY., Shon JH., Kim KA., Jung HJ., Shim JC., Yoon YR, et al. Combined effects of itraconazole and CYP2D6∗10 genetic polymorphism on the pharmacokinetics and pharmacodynamics of haloperidol in healthy subjects. J Clin Psychopharmacol. 2006. 26:135–142.
Article18.Pan LP., De Vriendt C., Belpaire FM. In-vitro characterization of the cytochrome P450 isoenzymes involved in the back oxidation and N-dealkylation of reduced haloperidol. Pharmacogenetics. 1998. 8:383–389.19.von Moltke LL., Greenblatt DJ., Schmider J., Harmatz JS., Shader RI. Metabolism of drugs by cytochrome P450 3A isoforms. Implications for drug interactions in psychopharmacology. Clin Pharmacokinet. 1995. 29:S33–S43. discussion 43-4.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Haloperidol and Clonidine Combination in the Treatment of Chronic Schizophrenia
- Simultaneous Determination of Four Compounds from Cercidiphyllum japonicum Using HPLC-UV Analysis
- Validation of High Performance Liquid Chromatographic Method with UV Detector for the Determination of Di(2-ethylhexyl)Phthalate in Plasma in some Korean Male Workers
- Plasma haloperidol, reduced haloperidol and homovanillic acid levels :therir relationship to therapeutic response of haloperidol in schizophtrenic patients
- HPLC-UV method for the simultaneous determinations of ascorbic acid and dehydroascorbic acid in human plasma