Korean J Radiol.
2016 Feb;17(1):151-158. 10.3348/kjr.2016.17.1.151.
A New Flow-Diverter (the FloWise): In-Vivo Evaluation in an Elastase-Induced Rabbit Aneurysm Model
- Affiliations
-
- 1Severance Integrated Research Institute of Cerebrovascular Disease, Severance Hospital, Yonsei University College of Medicine, Seoul 03722, Korea. bmoon21@hanmail.net
- 2Department of Radiology, Severance Hospital, Yonsei University College of Medicine, Seoul 03722, Korea.
- KMID: 2351175
- DOI: http://doi.org/10.3348/kjr.2016.17.1.151
Abstract
OBJECTIVE
We aimed to evaluate the efficacy and safety of a newly developed, partially retrievable flow-diverter (the FloWise) in an elastase-induced rabbit aneurysm model.
MATERIALS AND METHODS
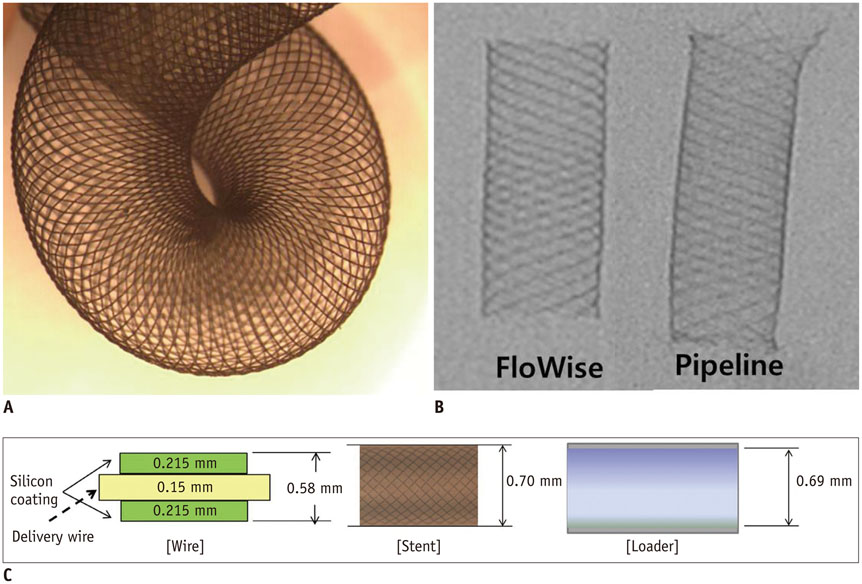
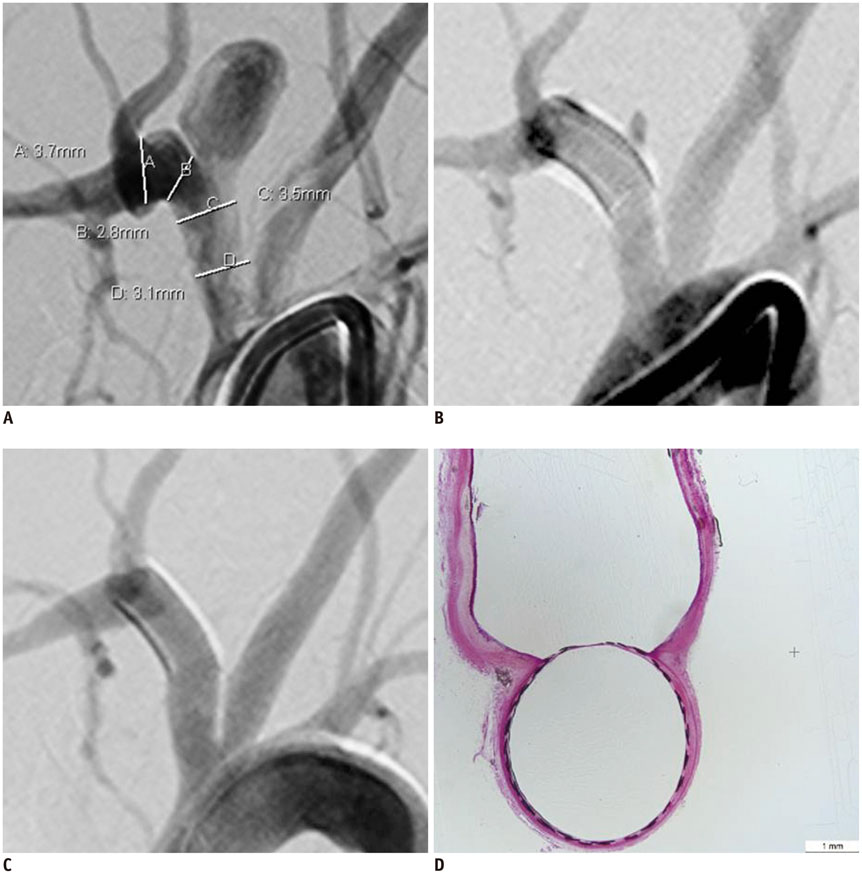
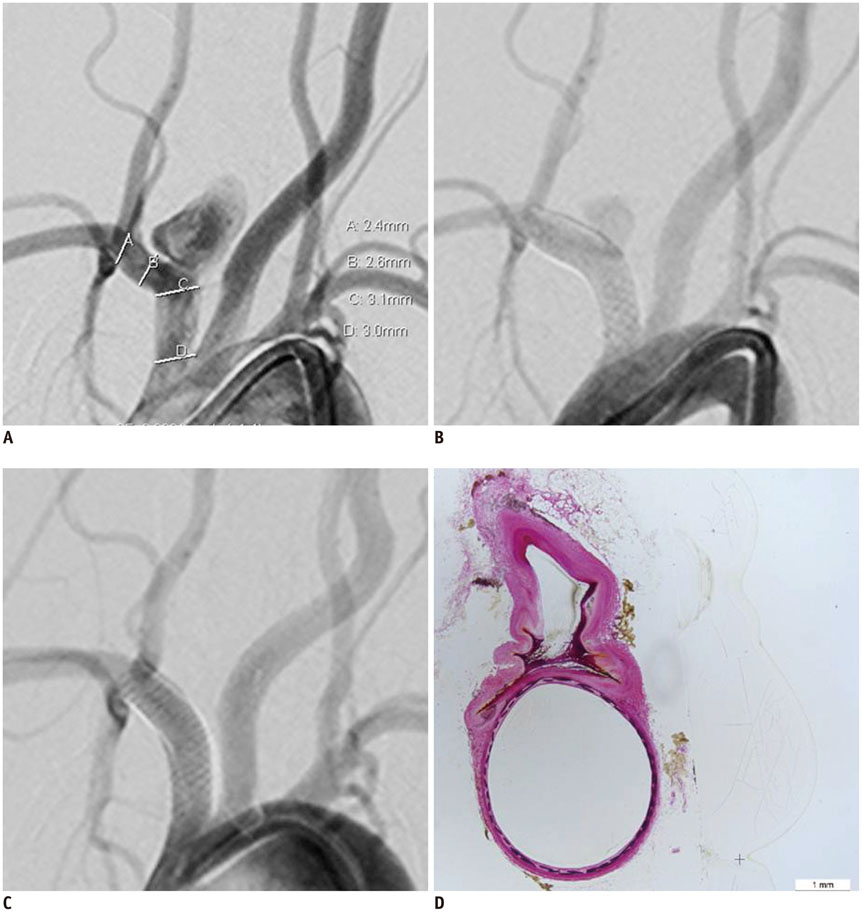
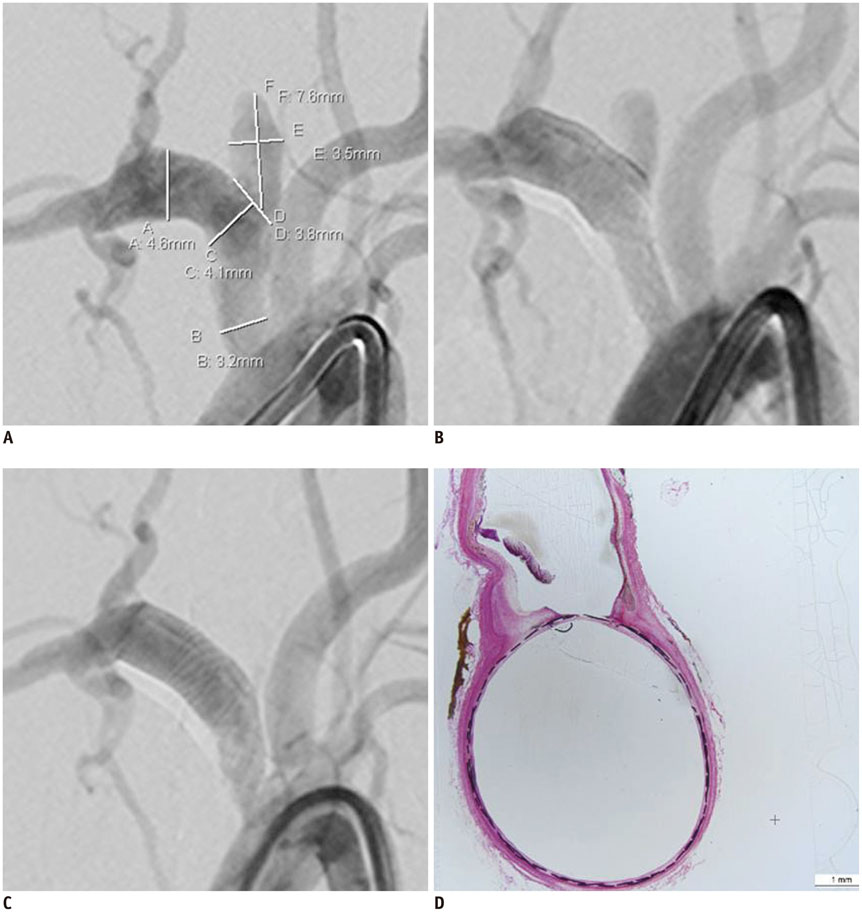
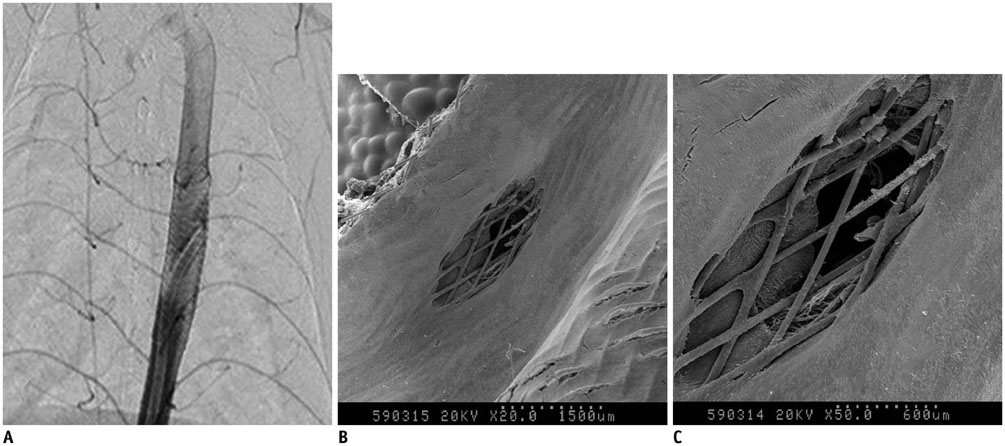
We developed a partially retrievable flow diverter composed of 48 strands of Nitinol and platinum wire. The FloWise is compatible with any microcatheter of 0.027-inch inner diameter, and is retrievable up to 70% deployment. The efficacy and safety of the FloWise were evaluated in the elastase-induced rabbit aneurysm model. The rate of technical success (full coverage of aneurysm neck) and assessment of aneurysm occlusion and stent patency was conducted by angiograms and histologic examinations at the 1-month, 3-month, and 6-month follow-up. The patency of small arterial branches (intercostal or lumbar arteries) covered by the FloWise were also assessed in the 5 subjects.
RESULTS
We attempted FloWise insertion in a total of 32 aneurysm models. FloWise placement was successful in 31 subjects (96.9%). Two stents (6.2%) were occluded at the 3-month follow-up, but there was no evidence of in-stent stenosis in other subjects. All stented aneurysms showed progressive occlusion: grade I (complete aneurysm occlusion) in 44.4% and grade II (aneurysm occlusion > 90%) in 55.6% at 1 month; grade I in 90% and II in 10% at 3 months; and grade I in 90% and II in 10% at 6 months. All small arterial branches covered by the FloWise remained patent.
CONCLUSION
A newly developed, partially retrievable flow-diverter seems to be a safe and effective tool of aneurysm occlusion, as evaluated in the rabbit aneurysm model.
Keyword
MeSH Terms
-
Alloys
Aneurysm/*chemically induced/radiography/*surgery
Angiography
Animals
Arteries/pathology/surgery
Catheters
Cerebrovascular Circulation/physiology
Constriction, Pathologic/chemically induced/radiography/surgery
*Disease Models, Animal
Humans
Male
Pancreatic Elastase/*pharmacology
Platinum
*Rabbits
Stents/*adverse effects
Alloys
Pancreatic Elastase
Platinum
Figure
Cited by 2 articles
-
A Novel Flow Diverter (Tubridge) for the Treatment of Recurrent Aneurysms: A Single-Center Experience
Yongxin Zhang, Qing-Hai Huang, Yibin Fang, Pengfei Yang, Yi Xu, Bo Hong, Jianmin Liu
Korean J Radiol. 2017;18(5):852-859. doi: 10.3348/kjr.2017.18.5.852.A Newly-Developed Flow Diverter (FloWise) for Internal Carotid Artery Aneurysm: Results of a Pilot Clinical Study
Byung Moon Kim, Keun Young Park, Jae Whan Lee, Joonho Chung, Dong Joon Kim, Dong Ik Kim
Korean J Radiol. 2019;20(3):505-512. doi: 10.3348/kjr.2018.0421.
Reference
-
1. Altes TA, Cloft HJ, Short JG, DeGast A, Do HM, Helm GA, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. American Roentgen Ray Society. AJR Am J Roentgenol. 2000; 174:349–354.2. Krings T, Möller-Hartmann W, Hans FJ, Thiex R, Brunn A, Scherer K, et al. A refined method for creating saccular aneurysms in the rabbit. Neuroradiology. 2003; 45:423–429.3. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007; 38:2346–2352.4. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A second-generation, endoluminal, flow-disrupting device for treatment of saccular aneurysms. AJNR Am J Neuroradiol. 2009; 30:1153–1158.5. Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna HR, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009; 64:632–642. discussion 642-643quiz N66. Lubicz B, Collignon L, Raphaeli G, Pruvo JP, Bruneau M, De Witte O, et al. Flow-diverter stent for the endovascular treatment of intracranial aneurysms: a prospective study in 29 patients with 34 aneurysms. Stroke. 2010; 41:2247–2253.7. Tähtinen OI, Manninen HI, Vanninen RL, Seppänen J, Niskakangas T, Rinne J, et al. The silk flow-diverting stent in the endovascular treatment of complex intracranial aneurysms: technical aspects and midterm results in 24 consecutive patients. Neurosurgery. 2012; 70:617–623. discussion 623-6248. Yu SC, Kwok CK, Cheng PW, Chan KY, Lau SS, Lui WM, et al. Intracranial aneurysms: midterm outcome of pipeline embolization device--a prospective study in 143 patients with 178 aneurysms. Radiology. 2012; 265:893–901.9. Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013; 44:442–447.10. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. 2013; 267:858–868.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Kinking of Flow Diverter in a Giant Wide-Necked Supraclinoid Internal Carotid Artery Aneurysm
- A Newly-Developed Flow Diverter (FloWise) for Internal Carotid Artery Aneurysm: Results of a Pilot Clinical Study
- Symptomatic Post Endarterectomy Common Carotid Artery Pseudoaneurysm Treated with Combination of Flow Diverter Implantation and Carotid Stenting
- Delayed Proximal Flow Diverting Stent Migration in a Ruptured Intracranial Aneurysm: A Case Report
- Clipping of a persistent middle cerebral artery aneurysm after previous flow diverter placement: An illustrative case and review of the literature