Yonsei Med J.
2015 Nov;56(6):1678-1685. 10.3349/ymj.2015.56.6.1678.
Resveratrol Inhibits Hypoxia-Induced Vascular Endothelial Growth Factor Expression and Pathological Neovascularization
- Affiliations
-
- 1The Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine, Severance Hospital, Seoul, Korea.
- 2Department of Biotechnology, College of Life Science, CHA University, Seongnam, Korea. jhchung@cha.ac.kr
- 3Myung-gok Eye Research Institute, Konyang University College of Medicine, Kim's Eye Hospital, Seoul, Korea.
- KMID: 2345900
- DOI: http://doi.org/10.3349/ymj.2015.56.6.1678
Abstract
- PURPOSE
To investigate the effects of resveratrol on the expression of hypoxia-inducible factor 1alpha (HIF-1alpha) and vascular endothelial growth factor (VEGF) in human adult retinal pigment epithelial (ARPE-19) cells, and on experimental choroidal neovascularization (CNV) in mice.
MATERIALS AND METHODS
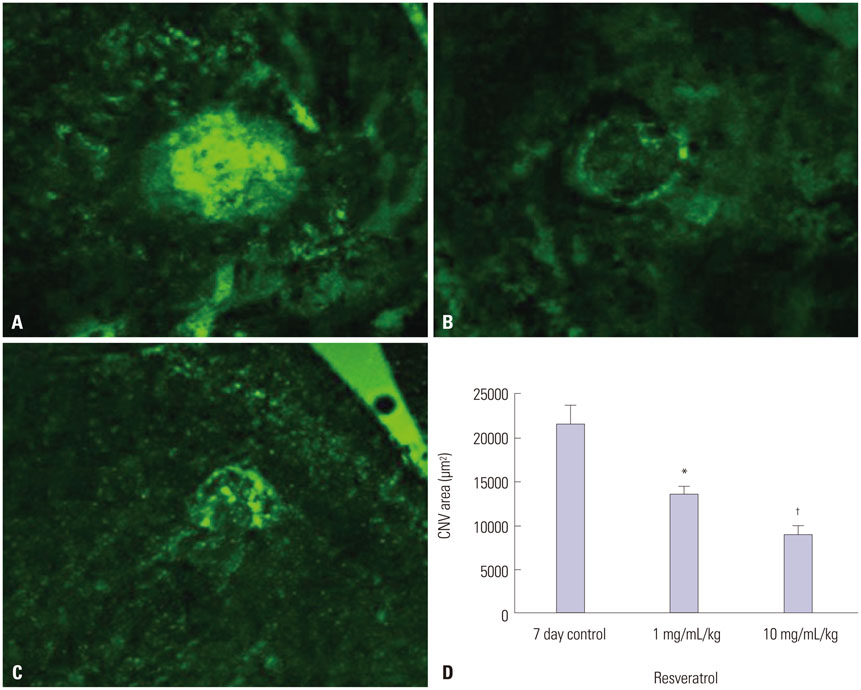
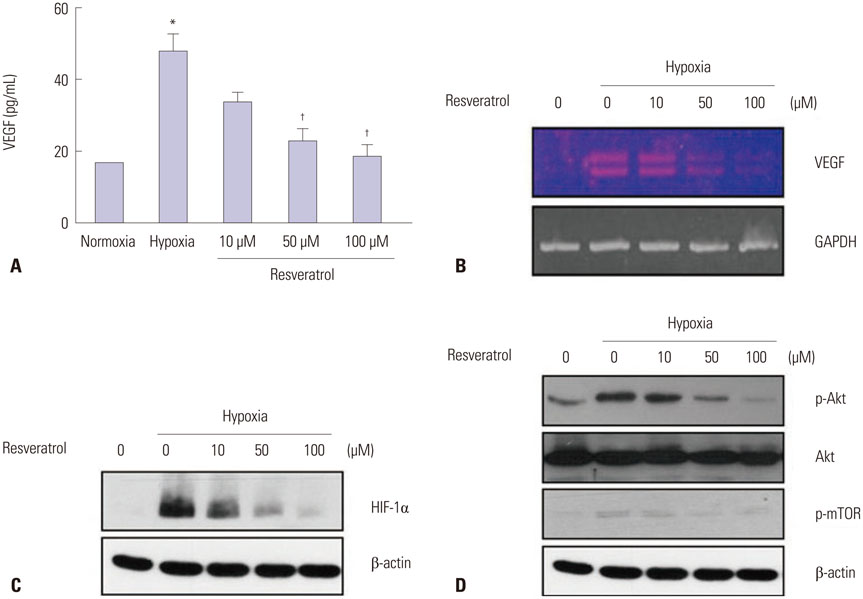
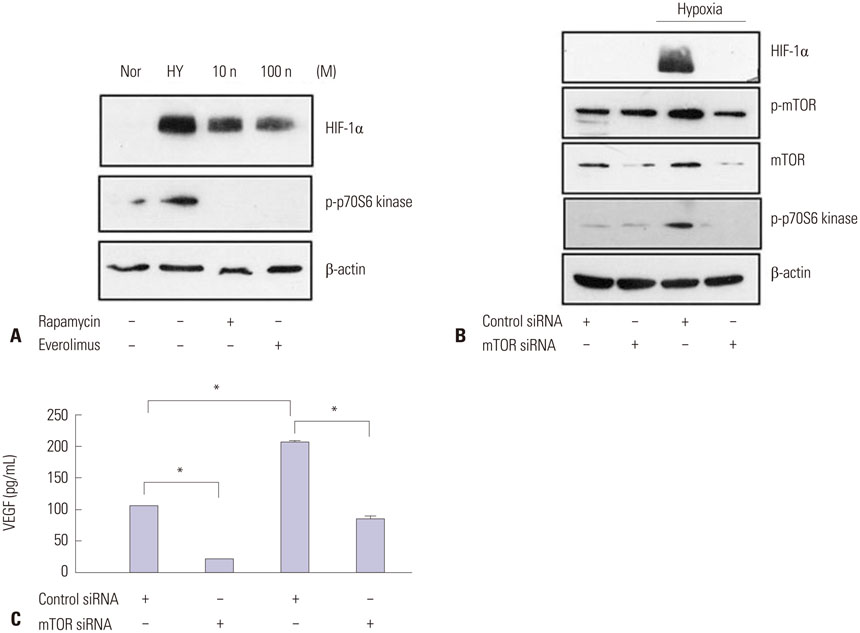
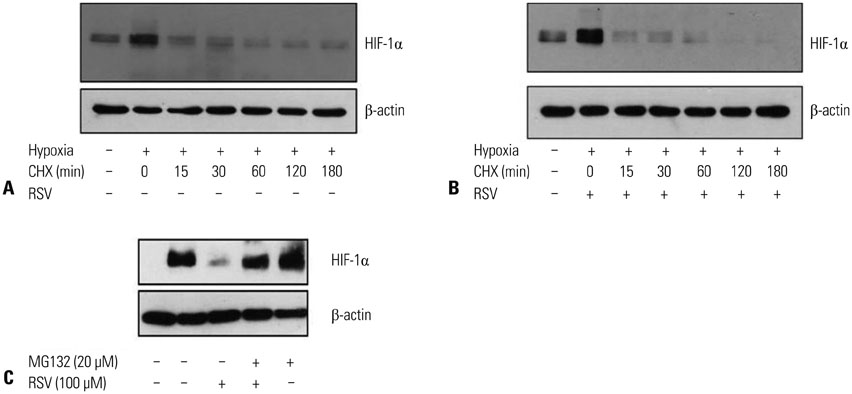
ARPE-19 cells were treated with different concentrations of resveratrol and then incubated under hypoxic conditions with subsequent evaluation of cell viability, expression of HIF-1alpha, and expression of VEGF. The effects of resveratrol on the synthesis and degradation of hypoxia-induced HIF-1alpha were evaluated using inhibitors of the PI3K/Akt/mTOR and the ubiquitin proteasome pathways. In animal studies, CNV lesions were induced in C57BL/6 mice by laser photocoagulation. After 7 days of oral administration of resveratrol or vehicle, which began one day after CNV induction, image analysis was used to measure CNV areas on choroidal flat mounts stained with isolectin IB4.
RESULTS
In ARPE-19 cells, resveratrol significantly inhibited HIF-1alpha and VEGF in a dose-dependent manner, by blocking the PI3K/Akt/mTOR signaling pathway and by promoting proteasomal HIF-1alpha degradation. In mice experiments, orally administered resveratrol significantly inhibited CNV growth in a dose-dependent manner.
CONCLUSION
Resveratrol may have therapeutic value in the management of diseases involving pathological neovascularization.
Keyword
MeSH Terms
-
Adult
Animals
Anoxia/metabolism/physiopathology
Cell Survival/drug effects
Choroidal Neovascularization/*metabolism/pathology
Humans
Hypoxia-Inducible Factor 1, alpha Subunit/*drug effects/metabolism
Mice
Mice, Inbred C57BL
Phosphatidylinositol 3-Kinases/antagonists & inhibitors/*physiology
Proteasome Endopeptidase Complex
Proto-Oncogene Proteins c-akt/antagonists & inhibitors/*physiology
Retinal Pigment Epithelium/*drug effects/metabolism
Signal Transduction
Stilbenes/administration & dosage/*pharmacology
TOR Serine-Threonine Kinases/antagonists & inhibitors/*physiology
Ubiquitin
Vascular Endothelial Growth Factor A/*drug effects/metabolism
Phosphatidylinositol 3-Kinases
Proteasome Endopeptidase Complex
Proto-Oncogene Proteins c-akt
Hypoxia-Inducible Factor 1, alpha Subunit
Stilbenes
TOR Serine-Threonine Kinases
Ubiquitin
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Hyman LG, Lilienfeld AM, Ferris FL 3rd, Fine SL. Senile macular degeneration: a case-control study. Am J Epidemiol. 1983; 118:213–227.
Article2. Mintz-Hittner HA, Kennedy KA, Chuang AZ. BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011; 364:603–615.
Article3. Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444.
Article4. Lux A, Llacer H, Heussen FM, Joussen AM. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol. 2007; 91:1318–1322.
Article5. Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010; 33:2399–2405.
Article6. Semenza GL. Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochem Pharmacol. 2000; 59:47–53.
Article7. Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985). 2000; 88:1474–1480.8. Inoue Y, Yanagi Y, Matsuura K, Takahashi H, Tamaki Y, Araie M. Expression of hypoxia-inducible factor 1alpha and 2alpha in choroidal neovascular membranes associated with age-related macular degeneration. Br J Ophthalmol. 2007; 91:1720–1721.
Article9. Sheridan CM, Pate S, Hiscott P, Wong D, Pattwell DM, Kent D. Expression of hypoxia-inducible factor-1alpha and -2alpha in human choroidal neovascular membranes. Graefes Arch Clin Exp Ophthalmol. 2009; 247:1361–1367.10. Juhasz B, Varga B, Gesztelyi R, Kemeny-Beke A, Zsuga J, Tosaki A. Resveratrol: a multifunctional cytoprotective molecule. Curr Pharm Biotechnol. 2010; 11:810–818.
Article11. Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997; 275:218–220.
Article12. Zhang Q, Tang X, Lu QY, Zhang ZF, Brown J, Le AD. Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1alpha and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells. Mol Cancer Ther. 2005; 4:1465–1474.
Article13. Cao Z, Fang J, Xia C, Shi X, Jiang BH. trans-3,4,5'-Trihydroxystibene inhibits hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clin Cancer Res. 2004; 10:5253–5263.
Article14. Park SY, Jeong KJ, Lee J, Yoon DS, Choi WS, Kim YK, et al. Hypoxia enhances LPA-induced HIF-1alpha and VEGF expression: their inhibition by resveratrol. Cancer Lett. 2007; 258:63–69.
Article15. Chung EJ, Yoo S, Lim HJ, Byeon SH, Lee JH, Koh HJ. Inhibition of choroidal neovascularisation in mice by systemic administration of the multikinase inhibitor, sorafenib. Br J Ophthalmol. 2009; 93:958–963.
Article16. Kang S, Roh YJ, Kim IB. Antiangiogenic effects of tivozanib, an oral VEGF receptor tyrosine kinase inhibitor, on experimental choroidal neovascularization in mice. Exp Eye Res. 2013; 112:125–133.
Article17. Kang S, Park KC, Yang KJ, Choi HS, Kim SH, Roh YJ. Effect of cediranib, an inhibitor of vascular endothelial growth factor receptor tyrosine kinase, in a mouse model of choroidal neovascularization. Clin Experiment Ophthalmol. 2013; 41:63–72.
Article18. Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002; 64:993–998.
Article19. Minet E, Michel G, Mottet D, Raes M, Michiels C. Transduction pathways involved in Hypoxia-Inducible Factor-1 phosphorylation and activation. Free Radic Biol Med. 2001; 31:847–855.
Article20. Jiang H, Shang X, Wu H, Gautam SC, Al-Holou S, Li C, et al. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J Exp Ther Oncol. 2009; 8:25–33.21. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003; 3:721–732.
Article22. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006; 444:337–342.
Article23. Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005; 39:813–822.
Article24. Balaiya S, Murthy RK, Chalam KV. Resveratrol inhibits proliferation of hypoxic choroidal vascular endothelial cells. Mol Vis. 2013; 19:2385–2392.25. Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998; 95:7987–7992.
Article26. Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, et al. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010; 51:2813–2826.
Article27. Khan AA, Dace DS, Ryazanov AG, Kelly J, Apte RS. Resveratrol regulates pathologic angiogenesis by a eukaryotic elongation factor-2 kinase-regulated pathway. Am J Pathol. 2010; 177:481–492.
Article28. Kanavi MR, Darjatmoko S, Wang S, Azari AA, Farnoodian M, Kenealey JD, et al. The sustained delivery of resveratrol or a defined grape powder inhibits new blood vessel formation in a mouse model of choroidal neovascularization. Molecules. 2014; 19:17578–17603.
Article29. Kim YH, Kim YS, Roh GS, Choi WS, Cho GJ. Resveratrol blocks diabetes-induced early vascular lesions and vascular endothelial growth factor induction in mouse retinas. Acta Ophthalmol. 2012; 90:e31–e37.
Article30. Hua J, Guerin KI, Chen J, Michán S, Stahl A, Krah NM, et al. Resveratrol inhibits pathologic retinal neovascularization in Vldlr(-/-) mice. Invest Ophthalmol Vis Sci. 2011; 52:2809–2816.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Hypoxia-Inducible Factor-1alpha(HIF-1alpha) in the Experimental Model of Choroidal Neovascularization
- The discovery of placenta growth factor and its biological activity
- The Effects of Resveratrol via Mediation of Nitric Oxide Synthase (NOS) on Hypoxic Retinal Injury in Neonatal Rats
- Thymosin Beta-4, Actin-Sequestering Protein Regulates Vascular Endothelial Growth Factor Expression via Hypoxia-Inducible Nitric Oxide Production in HeLa Cervical Cancer Cells
- Effect of Resveratrol on Oral Cancer Cell Invasion Induced by Lysophosphatidic Acid