Yonsei Med J.
2015 Nov;56(6):1590-1596. 10.3349/ymj.2015.56.6.1590.
The Role of Foxo3 in Leydig Cells
- Affiliations
-
- 1Endocrinology, Institute of Endocrine Research, Brain Korea 21 PLUS Project for Medical Science and Yonsei University College of Medicine, Seoul, Korea. EJLEE423@yuhs.ac
- 2Department of Internal Medicine, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea.
- 3Endocrinology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
- KMID: 2345887
- DOI: http://doi.org/10.3349/ymj.2015.56.6.1590
Abstract
- PURPOSE
Foxo3 in female reproduction has been reported to regulate proliferation of granulose cells that form follicles. There are no reports so far that discuss on the role of Foxo3 in males. This study was designed to outline the role of Foxo3 in the testes.
MATERIALS AND METHODS
Testes from mice at birth to postpartum week (PPW) 5 were isolated and examined for the expression of Foxo3 using immunostaining. To elucidate role of Foxo3 in Leydig cells, R2C cells were treated with luteinizing hormone (LH) and the phosphorylation of Foxo3. Testosterone and steroidogenic acute regulatory (StAR) protein levels were measured after constitutive active [triple mutant (TM)] human FOXO3 adenovirus was transduced and StAR promoter assay was performed.
RESULTS
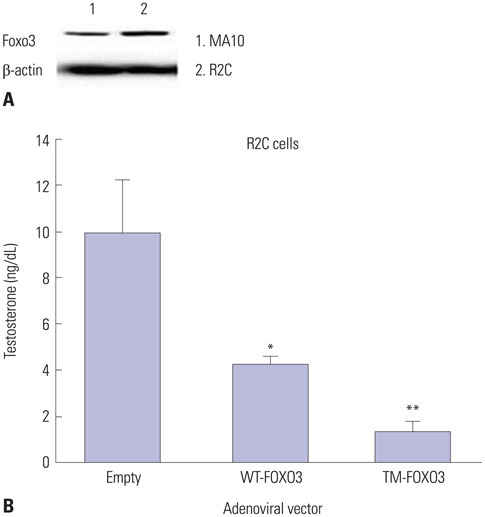
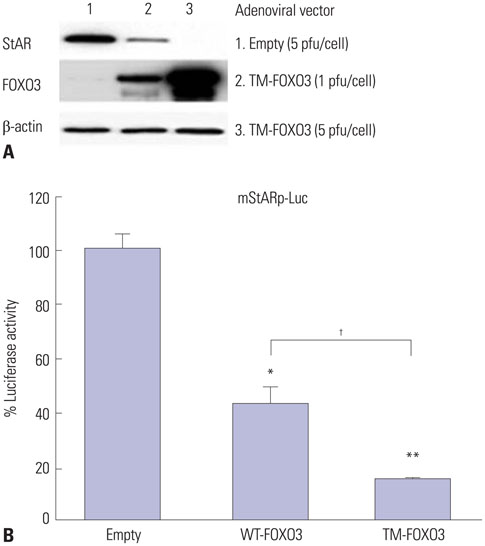
Foxo3 expression in the testicles started from birth and lasted until PPW 3. After PPW 3, most Foxo3 expression occurred in the nuclei of Leydig cells; however, at PPW 5, Foxo3 was expressed in both the nucleus and cytoplasm. When R2C cells were treated with luteinizing hormone, Foxo3 phosphorylation levels by AKT increased. After blocking the PI3K pathway, LH-induced phosphorylated Foxo3 levels decreased, indicating that LH signaling regulates Foxo3 localization. When active FOXO3-TM adenovirus was introduced into a Leydig tumor cell line, the concentrations of testosterone and StAR protein decreased. When FOXO3 and a StAR promoter vector were co-transfected into HEK293 cells for a reporter assay, FOXO3 inhibited the StAR promoter.
CONCLUSION
FOXO3 affects testosterone synthesis by inhibiting the formation of StAR protein. LH hormone, meanwhile, influences Foxo3 localization, mediating its function.
Keyword
MeSH Terms
-
Animals
Cell Aging/*physiology
Cell Nucleus/metabolism
Cytoplasm/metabolism
Forkhead Transcription Factors/*metabolism
HEK293 Cells
Humans
Leydig Cells/*drug effects/*enzymology/metabolism
Luteinizing Hormone/blood
Male
Mice
Phosphatidylinositol 3-Kinases
Phosphoproteins/metabolism
Phosphorylation
Signal Transduction/drug effects
Testosterone/blood/*metabolism
Forkhead Transcription Factors
Luteinizing Hormone
Phosphatidylinositol 3-Kinases
Phosphoproteins
Testosterone
Figure
Cited by 1 articles
-
Poorly-Controlled Type 1 Diabetes Mellitus Impairs LH-LHCGR Signaling in the Ovaries and Decreases Female Fertility in Mice
Jaewang Lee, Hoi Chang Lee, So-Youn Kim, Geum Joon Cho, Teresa K. Woodruff
Yonsei Med J. 2019;60(7):667-678. doi: 10.3349/ymj.2019.60.7.667.
Reference
-
1. Huhtaniemi I, Bartke A. Perspective: male reproduction. Endocrinology. 2001; 142:2178–2183.
Article2. Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003; 125:769–784.3. Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, Le-Meur M, et al. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A. 1998; 95:13612–13617.
Article4. Purvis K, Hansson V. Hormonal regulation of Leydig cell function. Mol Cell Endocrinol. 1978; 12:123–138.
Article5. Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci U S A. 2004; 101:17294–17299.
Article6. Jasti P, Dunaif A. Reproduction and metabolism: insights from polycystic ovary syndrome. Endocrinol Metab. 2012; 27:180. 190.
Article7. Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007; 120(Pt 15):2479–2487.8. Lee EJ, Anderson LM, Thimmapaya B, Jameson JL. Targeted expression of toxic genes directed by pituitary hormone promoters: a potential strategy for adenovirus-mediated gene therapy of pituitary tumors. J Clin Endocrinol Metab. 1999; 84:786–794.9. Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta. 2011; 1813:1938–1945.
Article10. Richards JS, Sharma SC, Falender AE, Lo YH. Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary: evidence for regulation by IGF-I, estrogen, and the gonadotropins. Mol Endocrinol. 2002; 16:580–599.11. Park Y, Maizels ET, Feiger ZJ, Alam H, Peters CA, Woodruff TK, et al. Induction of cyclin D2 in rat granulosa cells requires FSH-dependent relief from FOXO1 repression coupled with positive signals from Smad. J Biol Chem. 2005; 280:9135–9148.12. Hosaka T, Biggs WH 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, et al. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004; 101:2975–2980.
Article13. Matsuda F, Inoue N, Maeda A, Cheng Y, Sai T, Gonda H, et al. Expression and function of apoptosis initiator FOXO3 in granulosa cells during follicular atresia in pig ovaries. J Reprod Dev. 2011; 57:151–158.
Article14. Goertz MJ, Wu Z, Gallardo TD, Hamra FK, Castrillon DH. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J Clin Invest. 2011; 121:3456–3466.
Article15. Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003; 301:215–218.
Article16. Lee EJ, Kim JM, Lee MK, Jameson JL. Splice variants of the forkhead box protein AFX exhibit dominant negative activity and inhibit AFXalpha-mediated tumor cell apoptosis. PLoS One. 2008; 3:e2743.17. Potente M, Fisslthaler B, Busse R, Fleming I. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J Biol Chem. 2003; 278:29619–29625.
Article18. Hong ZY, Lee HJ, Shin DY, Kim SK, Seo M, Lee EJ. Inhibition of Akt/FOXO3a signaling by constitutively active FOXO3a suppresses growth of follicular thyroid cancer cell lines. Cancer Lett. 2012; 314:34–40.
Article19. Kim JW, Kim HS, Kim SD, Park JY. Insulin Phosphorylates Tyrosine Residue 464 of Tub and Translocates Tubby into the Nucleus in HIRcB Cells. Endocrinol Metab (Seoul). 2014; 29:163–168.20. Ketola I, Pentikäinen V, Vaskivuo T, Ilvesmäki V, Herva R, Dunkel L, et al. Expression of transcription factor GATA-4 during human testicular development and disease. J Clin Endocrinol Metab. 2000; 85:3925–3931.
Article21. Aspden WJ, Rodgers RJ, Stocco DM, Scott PT, Wreford NG, Trigg TE, et al. Changes in testicular steroidogenic acute regulatory (STAR) protein, steroidogenic enzymes and testicular morphology associated with increased testosterone secretion in bulls receiving the luteinizing hormone releasing hormone agonist deslorelin. Domest Anim Endocrinol. 1998; 15:227–238.
Article22. Wang XX, Wu ZY. [Prediction of ovulation]. Zhonghua Fu Chan Ke Za Zhi. 1990; 25:86–88. 12423. Bäckström CT, McNeilly AS, Leask RM, Baird DT. Pulsatile secretion of LH, FSH, prolactin, oestradiol and progesterone during the human menstrual cycle. Clin Endocrinol (Oxf). 1982; 17:29–42.
Article24. Erickson GF, Ryan KJ. Stimulation of testosterone production in isolated rabbit thecal tissue by LH/FSH, dibutyryl cyclic AMP, PGE2alpha, and PGE2. Endocrinology. 1976; 99:452–458.
Article25. Palaniappan M, Menon KM. Human chorionic gonadotropin stimulates theca-interstitial cell proliferation and cell cycle regulatory proteins by a cAMP-dependent activation of AKT/mTORC1 signaling pathway. Mol Endocrinol. 2010; 24:1782–1793.
Article26. Bogovich K, Richards JS. Androgen biosynthesis in developing ovarian follicles: evidence that luteinizing hormone regulates thecal 17 alpha-hydroxylase and C17-20-lyase activities. Endocrinology. 1982; 111:1201–1208.
Article27. Magoffin DA, Kurtz KM, Erickson GF. Insulin-like growth factor-I selectively stimulates cholesterol side-chain cleavage expression in ovarian theca-interstitial cells. Mol Endocrinol. 1990; 4:489–496.
Article28. Magoffin DA, Weitsman SR. Differentiation of ovarian theca-interstitial cells in vitro: regulation of 17 alpha-hydroxylase messenger ribonucleic acid expression by luteinizing hormone and insulin-like growth factor-I. Endocrinology. 1993; 132:1945–1951.
Article29. Magoffin DA, Weitsman SR. Insulin-like growth factor-I stimulates the expression of 3 beta-hydroxysteroid dehydrogenase messenger ribonucleic acid in ovarian theca-interstitial cells. Biol Reprod. 1993; 48:1166–1173.
Article30. Mizutani T, Yazawa T, Ju Y, Imamichi Y, Uesaka M, Inaoka Y, et al. Identification of a novel distal control region upstream of the human steroidogenic acute regulatory protein (StAR) gene that participates in SF-1-dependent chromatin architecture. J Biol Chem. 2010; 285:28240–28251.
Article31. Fukuda S, Orisaka M, Tajima K, Hattori K, Kotsuji F. Luteinizing hormone-induced Akt phosphorylation and androgen production are modulated by MAP Kinase in bovine theca cells. J Ovarian Res. 2009; 2:17.
Article32. Shiraishi K, Ascoli M. Lutropin/choriogonadotropin stimulate the proliferation of primary cultures of rat Leydig cells through a pathway that involves activation of the extracellularly regulated kinase 1/2 cascade. Endocrinology. 2007; 148:3214–3225.
Article33. Martinelle N, Holst M, Söder O, Svechnikov K. Extracellular signal-regulated kinases are involved in the acute activation of steroidogenesis in immature rat Leydig cells by human chorionic gonadotropin. Endocrinology. 2004; 145:4629–4634.
Article34. Stocco DM, Clark BJ. Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochem Pharmacol. 1996; 51:197–205.
Article35. Murayama C, Miyazaki H, Miyamoto A, Shimizu T. Luteinizing hormone (LH) regulates production of androstenedione and progesterone via control of histone acetylation of StAR and CYP17 promoters in ovarian theca cells. Mol Cell Endocrinol. 2012; 350:1–9.
Article36. Wang G, Hardy MP. Development of leydig cells in the insulin-like growth factor-I (igf-I) knockout mouse: effects of igf-I replacement and gonadotropic stimulation. Biol Reprod. 2004; 70:632–639.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Experimental Study on the Role of Leydig Cell in Testicular Descent
- Increase in Transepithelial Resistance Mouse Sertoli Cells by Leydig Cells Coculture
- Morphological Quantitative Study on Leydig Cell: Effect of Aging
- MiR-29a-3p Inhibits Proliferation and Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells via Targeting FOXO3 and Repressing Wnt/β-Catenin Signaling in Steroid-Associated Osteonecrosis
- Ovarian serous cystadenoma associated with Sertoli-Leydig cell tumor: a case report