Int J Stem Cells.
2022 Aug;15(3):324-333. 10.15283/ijsc21147.
MiR-29a-3p Inhibits Proliferation and Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells via Targeting FOXO3 and Repressing Wnt/β-Catenin Signaling in Steroid-Associated Osteonecrosis
- Affiliations
-
- 1Department of Emergency and Trauma Surgery, People’s Hospital of Pingxiang, Pingxiang, China
- KMID: 2532406
- DOI: http://doi.org/10.15283/ijsc21147
Abstract
- Background and Objectives
This study was to investigate the role of microRNA-29a-3p (miR-29a-3p) in human bone marrow mesenchymal stem cells (hBMSCs), and its relationship with steroid-associated osteonecrosis.
Methods and Results
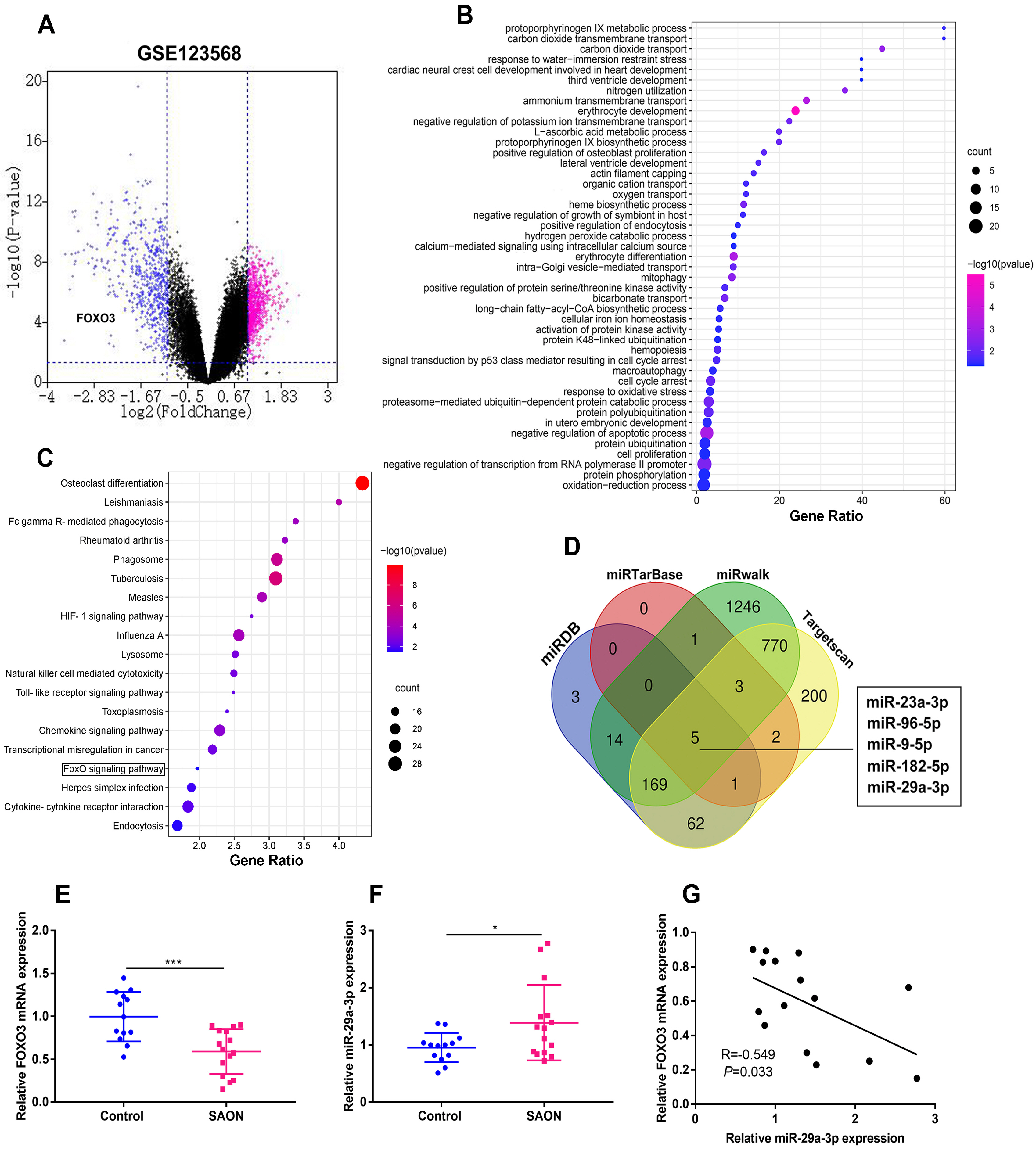
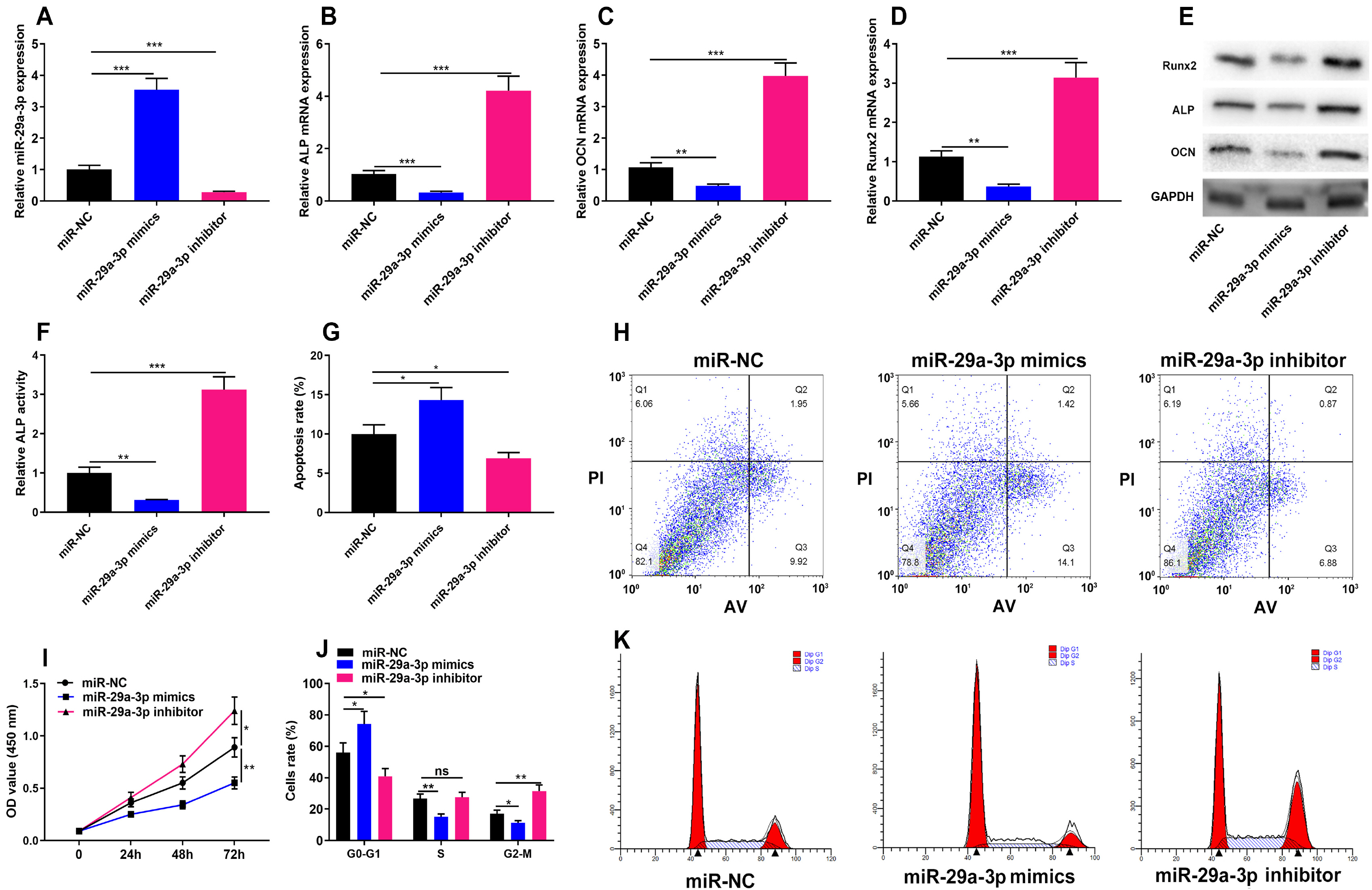
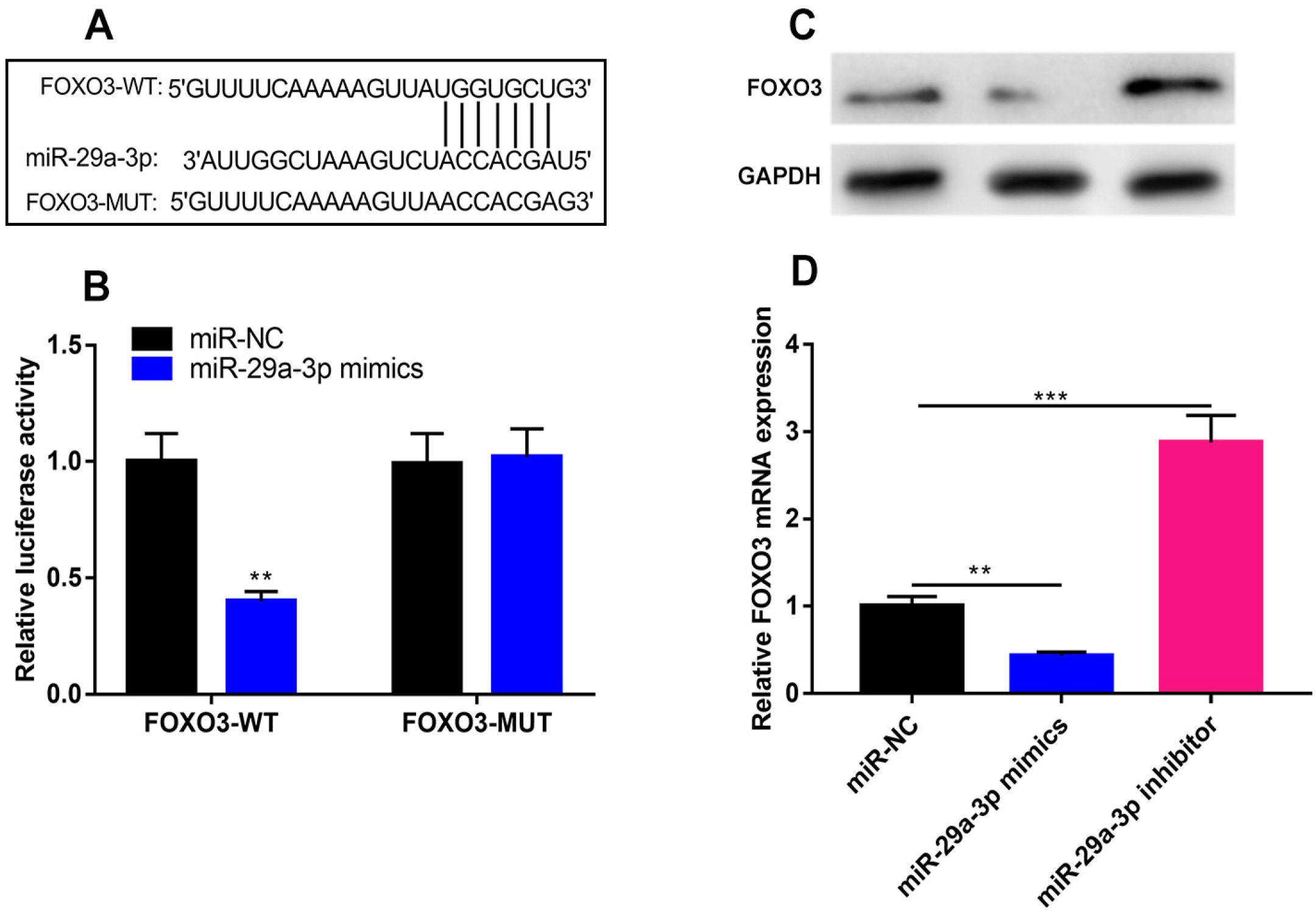
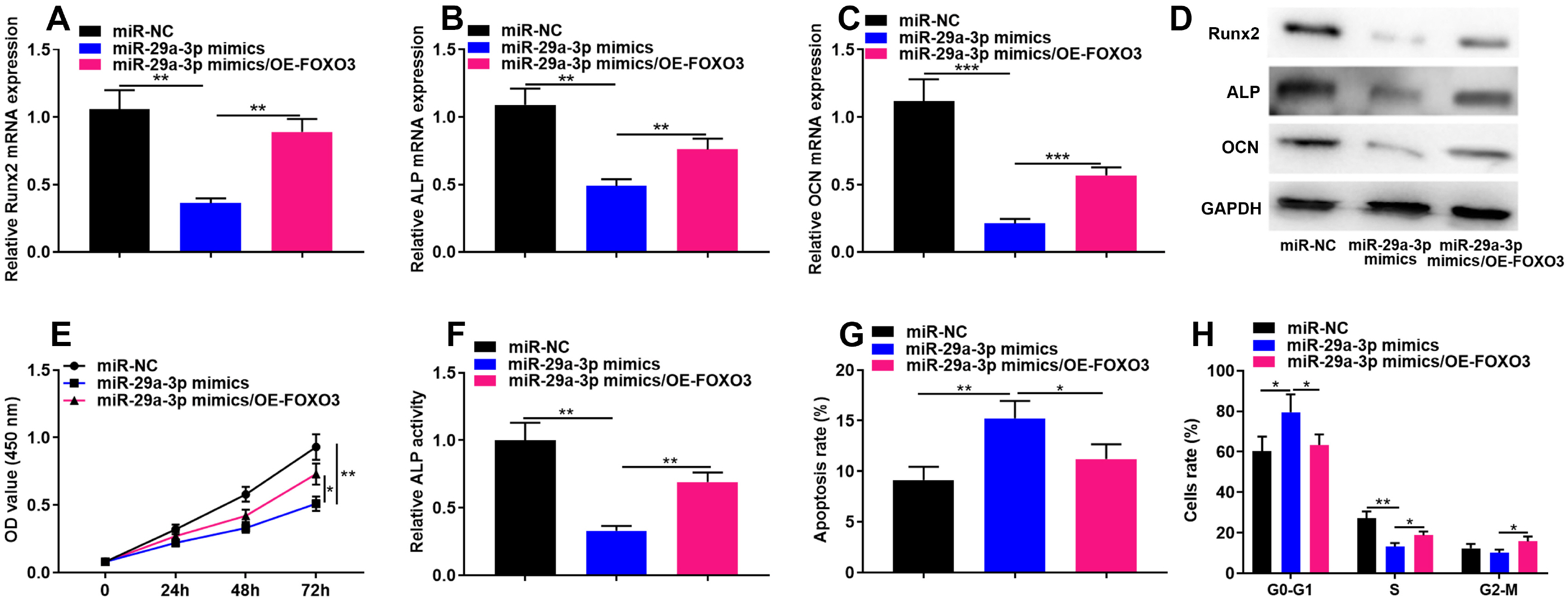
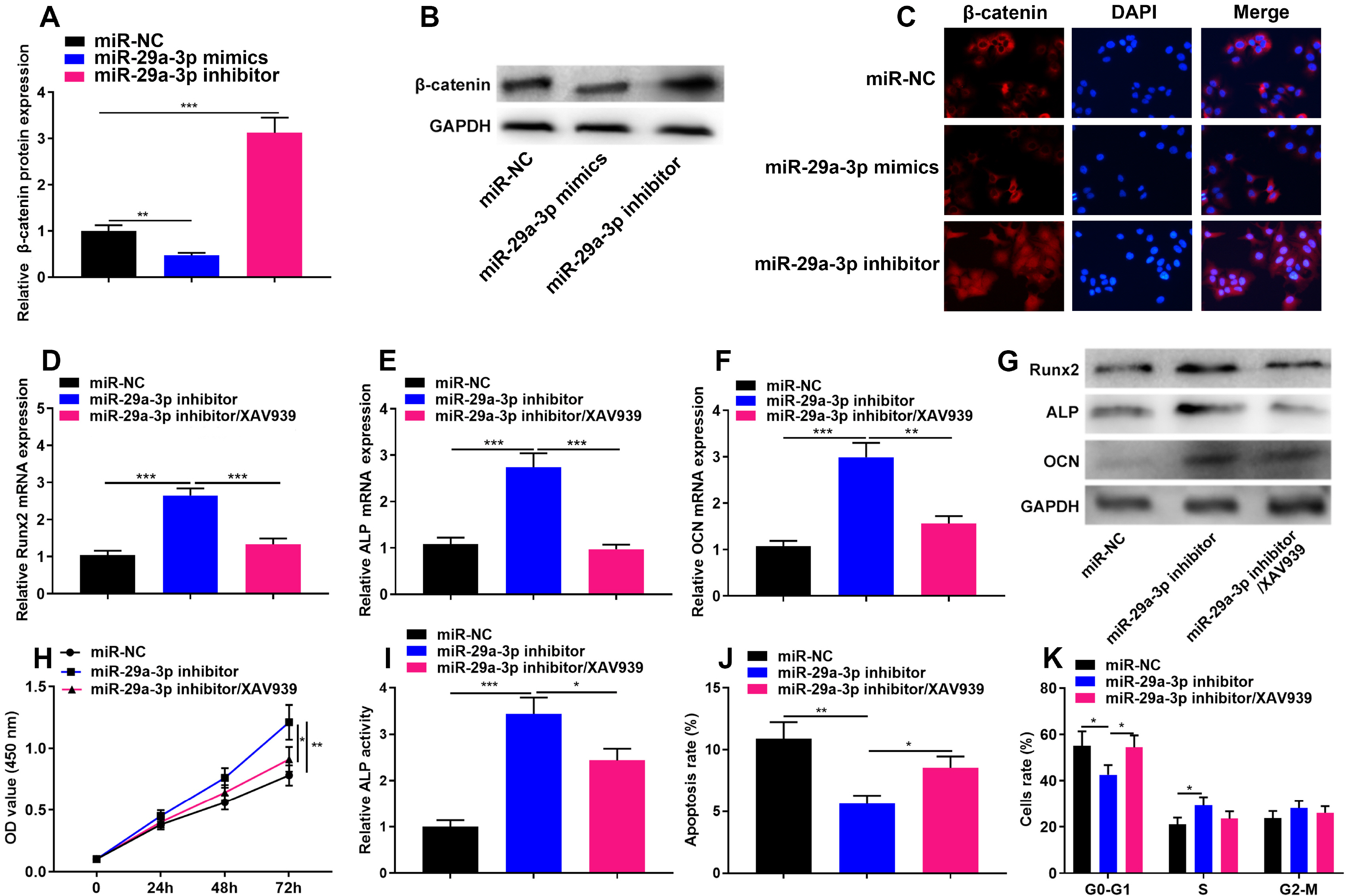
The online tool GEO2R was used to screen out the differentially expressed genes (DEGs) in GSE123568 dataset. Quantitative real time-polymerase chain reaction (qRT-PCR) was performed to detect the expression of miR-29a-3p, forkhead box O3 (FOXO3), alkaline phosphatase (ALP), bone gamma-carboxyglutamate protein (OCN) and RUNX family transcription factor 2 (Runx2) in the hBMSCs isolated from the patients with steroid-associated osteonecrosis. CCK-8 assay was executed to measure cell viability; western blot assay was utilized to detect FOXO3, ALP, Runx2, OCN and β-catenin expression. Cell apoptosis and cell cycle were detected by flow cytometry. Immunofluorescence assay was used to detect the sub-cellular localization of β-catenin. Bioinformatics analysis and luciferase reporter gene assay were performed to confirm whether miR-29a-3p can combine with FOXO3 3’UTR. MiR-29a-3p was markedly up-regulated in the hBMSCs of patients with steroid-associated osteonecrosis, while FOXO3 mRNA was significantly down-regulated. Transfection of miR-29a-3p mimics significantly inhibited the hBMSCs’ proliferation, osteogenic differentiation markers’ expressions, including ALP, Runx2, OCN, and repressed the ALP activity, as well as promoted cell apoptosis and cell-cycle arrest. FOXO3 was identified as a target gene of miR-29a-3p, and miR-29a-3p can inhibit the expression of FOXO3 and β-catenin, and inhibition of miR-29a-3p promoted translocation of β-catenin to the nucleus.
Conclusions
MiR-29a-3p can modulate FOXO3 expression and Wnt/β-catenin signaling to inhibit viability and osteogenic differentiation of hBMSCs, thereby promoting the development of steroid-associated osteonecrosis.
Figure
Reference
-
References
1. Bose VC, Baruah BD. 2010; Resurfacing arthroplasty of the hip for avascular necrosis of the femoral head: a minimum follow-up of four years. J Bone Joint Surg Br. 92:922–928. DOI: 10.1302/0301-620X.92B7.23639. PMID: 20595108. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77954245222&origin=inward.2. Yue J, Wan F, Zhang Q, Wen P, Cheng L, Li P, Guo W. 2018; Effect of glucocorticoids on miRNA expression spectrum of rat femoral head microcirculation endothelial cells. Gene. 651:126–133. DOI: 10.1016/j.gene.2018.01.057. PMID: 29408208. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85043276502&origin=inward.3. Su P, Tian Y, Yang C, Ma X, Wang X, Pei J, Qian A. 2018; Mesenchymal stem cell migration during bone formation and bone diseases therapy. Int J Mol Sci. 19:2343. DOI: 10.3390/ijms19082343. PMID: 30096908. PMCID: PMC6121650. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85052054845&origin=inward.4. Park KR, Kim S, Cho M, Kang SW, Yun HM. 2020; Effects of PIN on osteoblast differentiation and matrix mineralization through Runt-related transcription factor. Int J Mol Sci. 21:9579. DOI: 10.3390/ijms21249579. PMID: 33339165. PMCID: PMC7765567. PMID: 1e45d0759f72443a921f4b5f22724fa0. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85098109235&origin=inward.5. Deng S, Dai G, Chen S, Nie Z, Zhou J, Fang H, Peng H. 2019; Dexamethasone induces osteoblast apoptosis through ROS-PI3K/AKT/GSK3β signaling pathway. Biomed Pharmacother. 110:602–608. DOI: 10.1016/j.biopha.2018.11.103. PMID: 30537677. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85057760403&origin=inward.6. Beyer C, Zampetaki A, Lin NY, Kleyer A, Perricone C, Iagnocco A, Distler A, Langley SR, Gelse K, Sesselmann S, Lorenzini R, Niemeier A, Swoboda B, Distler JH, Santer P, Egger G, Willeit J, Mayr M, Schett G, Kiechl S. 2015; Signature of circulating microRNAs in osteoarthritis. Ann Rheum Dis. 74:e18. DOI: 10.1136/annrheumdis-2013-204698. PMID: 24515954. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84926613424&origin=inward.7. Wang J, Liu S, Li J, Zhao S, Yi Z. 2019; Roles for miRNAs in osteogenic differentiation of bone marrow mesenchymal stem cells. Stem Cell Res Ther. 10:197. DOI: 10.1186/s13287-019-1309-7. PMID: 31253175. PMCID: PMC6599379. PMID: 9f229def334e466eaf997a13b7c5877f. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85068114948&origin=inward.8. Liu Y, Zong Y, Shan H, Lin Y, Xia W, Wang N, Zhou L, Gao Y, Ma X, Jiang C. 2020; MicroRNA-23b-3p participates in steroid-induced osteonecrosis of the femoral head by suppressing ZNF667 expression. Steroids. 163:108709. DOI: 10.1016/j.steroids.2020.108709. PMID: 32730776. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85088986495&origin=inward.9. Zhao JJ, Wu ZF, Wang L, Feng DH, Cheng L. 2016; MicroRNA-145 mediates steroid-induced necrosis of the femoral head by targeting the OPG/RANK/RANKL signaling pathway. PLoS One. 11:e0159805. DOI: 10.1371/journal.pone.0159805. PMID: 27459539. PMCID: PMC4961289. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84979967833&origin=inward.10. Dai Z, Jin Y, Zheng J, Liu K, Zhao J, Zhang S, Wu F, Sun Z. 2019; MiR-217 promotes cell proliferation and osteogenic differentiation of BMSCs by targeting DKK1 in steroid-associated osteonecrosis. Biomed Pharmacother. 109:1112–1119. DOI: 10.1016/j.biopha.2018.10.166. PMID: 30551361. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85056182065&origin=inward.11. Jin H, Wang H, Jin X, Wang W. 2021; Long non-coding RNA H19 regulates LASP1 expression in osteosarcoma by competitively binding to miR-29a-3p. Oncol Rep. 46:207. DOI: 10.3892/or.2021.8158. PMID: 34328197. PMCID: PMC8329914. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85112712509&origin=inward.12. Muluhngwi P, Klinge CM. 2021; Identification and roles of miR-29b-1-3p and miR29a-3p-regulated and non-regulated lncRNAs in endocrine-sensitive and resistant breast cancer cells. Cancers (Basel). 13:3530. DOI: 10.3390/cancers13143530. PMID: 34298743. PMCID: PMC8307416. PMID: 1191218e3da64855b3b49f568268462a. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85109968760&origin=inward.13. Geng A, Luo L, Ren F, Zhang L, Zhou H, Gao X. 2021; miR-29a-3p inhibits endometrial cancer cell proliferation, migration and invasion by targeting VEGFA/CD C42/PAK1. BMC Cancer. 21:843. DOI: 10.1186/s12885-021-08506-z. PMID: 34289832. PMCID: PMC8293590. PMID: 1460ada594f0413ebf22337a255dbb86. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85110964820&origin=inward.14. Zhou W, Li H, Shang S, Liu F. 2021; lncRNA KCNQ1OT1 reverses the effect of sevoflurane on hepatocellular carcinoma progression via regulating the miR-29a-3p/CBX3 axis. Braz J Med Biol Res. 54:e10213. DOI: 10.1590/1414-431x2020e10213. PMID: 34008749. PMCID: PMC8130105. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85106181731&origin=inward.15. Salih DA, Brunet A. 2008; FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 20:126–136. DOI: 10.1016/j.ceb.2008.02.005. PMID: 18394876. PMCID: PMC2387118. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=41549135942&origin=inward.16. Gómez-Puerto MC, Verhagen LP, Braat AK, Lam EW, Coffer PJ, Lorenowicz MJ. 2016; Activation of autophagy by FOXO3 regulates redox homeostasis during osteogenic differentiation. Autophagy. 12:1804–1816. DOI: 10.1080/15548627.2016.1203484. PMID: 27532863. PMCID: PMC5079670. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84987677252&origin=inward.17. Ambrogini E, Almeida M, Martin-Millan M, Paik JH, Depinho RA, Han L, Goellner J, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC. 2010; FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 11:136–146. DOI: 10.1016/j.cmet.2009.12.009. PMID: 20142101. PMCID: PMC2819984. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=75149146587&origin=inward.18. Han S, Wang Z, Liu J, Wang HD, Yuan Q. 2021; miR-29a-3p-dependent COL3A1 and COL5A1 expression reduction assists sulforaphane to inhibit gastric cancer progression. Biochem Pharmacol. 188:114539. DOI: 10.1016/j.bcp.2021.114539. PMID: 33819468. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85105347226&origin=inward.19. Liu F, Wang Z, Liu F, Xu J, Liu Q, Yin K, Lan J. 2018; MicroRNA-29a-3p enhances dental implant osseointegration of hyperlipidemic rats via suppressing dishevelled 2 and frizzled 4. Cell Biosci. 8:55. DOI: 10.1186/s13578-018-0254-y. PMID: 30386554. PMCID: PMC6203977. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85055819139&origin=inward.20. Zeng X, Wang Y, Dong Q, Ma MX, Liu XD. 2020; DLX2 activates Wnt1 transcription and mediates Wnt/β-catenin signal to promote osteogenic differentiation of hBMSCs. Gene. 744:144564. DOI: 10.1016/j.gene.2020.144564. PMID: 32165291. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85082645341&origin=inward.21. Wu X, Sun W, Tan M. 2019; Noncoding RNAs in steroid-induced osteonecrosis of the femoral head. Biomed Res Int. 2019:8140595. DOI: 10.1155/2019/8140595. PMID: 31930139. PMCID: PMC6942769. PMID: df75567403834b33afc794072c95b9b6. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85077759117&origin=inward.22. Chen Y, Zhang W, Yan L, Zheng P, Li J. 2020; miR-29a-3p directly targets Smad nuclear interacting protein 1 and inhibits the migration and proliferation of cervical cancer HeLa cells. PeerJ. 8:e10148. DOI: 10.7717/peerj.10148. PMID: 33150075. PMCID: PMC7583608. PMID: c02ae6d368db472283f3ea771658137a. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85094101826&origin=inward.23. Zhu D, Tian J, Wu X, Li M, Tang X, Rui K, Guo H, Ma J, Xu H, Wang S. 2019; G-MDSC-derived exosomes attenuate collagen-induced arthritis by impairing Th1 and Th17 cell responses. Biochim Biophys Acta Mol Basis Dis. 1865:165540. DOI: 10.1016/j.bbadis.2019.165540. PMID: 31470074. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85071954017&origin=inward.24. Chen D, Gong Y, Xu L, Zhou M, Li J, Song J. 2019; Bidirectional regulation of osteogenic differentiation by the FOXO subfamily of Forkhead transcription factors in mammalian MSCs. Cell Prolif. 52:e12540. DOI: 10.1111/cpr.12540. PMID: 30397974. PMCID: PMC6496202. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85056200564&origin=inward.25. Long C, Cen S, Zhong Z, Zhou C, Zhong G. 2021; FOXO3 is targeted by miR-223-3p and promotes osteogenic differentiation of bone marrow mesenchymal stem cells by enhancing autophagy. Hum Cell. 34:14–27. DOI: 10.1007/s13577-020-00421-y. PMID: 32920731. PMCID: PMC7788031. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85090966235&origin=inward.26. Guérit D, Brondello JM, Chuchana P, Philipot D, Toupet K, Bony C, Jorgensen C, Noël D. 2014; FOXO3A regulation by miRNA-29a controls chondrogenic differentiation of mesenchymal stem cells and cartilage formation. Stem Cells Dev. 23:1195–1205. DOI: 10.1089/scd.2013.0463. PMID: 24467486. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84901317768&origin=inward.27. Fan J, An X, Yang Y, Xu H, Fan L, Deng L, Li T, Weng X, Zhang J, Chunhua Zhao R. 2018; MiR-1292 targets FZD4 to regulate senescence and osteogenic differentiation of stem cells in TE/SJ/mesenchymal tissue system via the Wnt/β-catenin pathway. Aging Dis. 9:1103–1121. DOI: 10.14336/AD.2018.1110. PMID: 30574422. PMCID: PMC6284756. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85060499427&origin=inward.28. Tu X, Delgado-Calle J, Condon KW, Maycas M, Zhang H, Carlesso N, Taketo MM, Burr DB, Plotkin LI, Bellido T. 2015; Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc Natl Acad Sci U S A. 112:E478–E486. DOI: 10.1073/pnas.1409857112. PMID: 25605937. PMCID: PMC4321271. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84922309760&origin=inward.29. Pei J, Fan L, Nan K, Li J, Shi Z, Dang X, Wang K. 2017; Excessive activation of TLR4/NF-κB interactively suppresses the canonical Wnt/β-catenin pathway and induces SANFH in SD rats. Sci Rep. 7:11928. DOI: 10.1038/s41598-017-12196-8. PMID: 28931847. PMCID: PMC5607349. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85029627047&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MiR-148a-3p Regulates the Invasion and Odontoblastic Differentiation of Human Dental Pulp Stem Cells via the Wnt1/β-Catenin Pathway
- Transforming Growth Factor-β Signaling Inhibits the Osteogenic Differentiation of Mesenchymal Stem Cells via Activation of Wnt/β-Catenin Pathway
- Natural Products Targeting Wnt/β-catenin Signaling Pathway
- Intra-Articular Injection of miR-29a-3p of BMSCs Promotes Cartilage Self-Repairing and Alleviates Pain in the Rat Osteoarthritis
- MiR-214 Regulates the Human Hair Follicle Stem Cell Proliferation and Differentiation by Targeting EZH2 and Wnt/β-Catenin Signaling Way In Vitro