Clin Nutr Res.
2016 Jul;5(3):172-179. 10.7762/cnr.2016.5.3.172.
Application of Iron Oxide as a pH-dependent Indicator for Improving the Nutritional Quality
- Affiliations
-
- 1Department of Food Science and Technology, Sejong University, Seoul 05006, Korea. sanghoonko@sejong.ac.kr
- 2School of Food Science, Kyungil University, Gyeongsan 38428, Korea.
- KMID: 2344889
- DOI: http://doi.org/10.7762/cnr.2016.5.3.172
Abstract
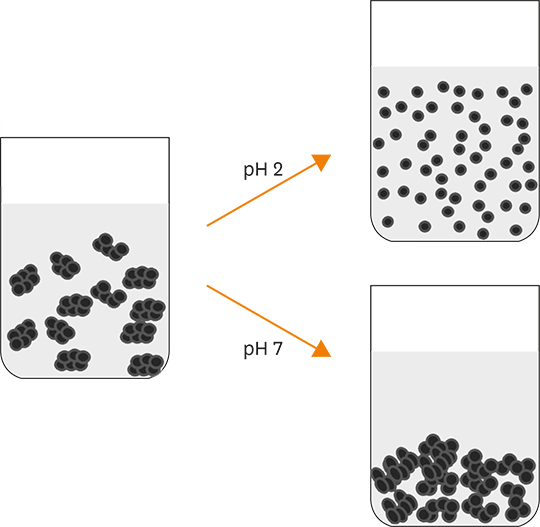
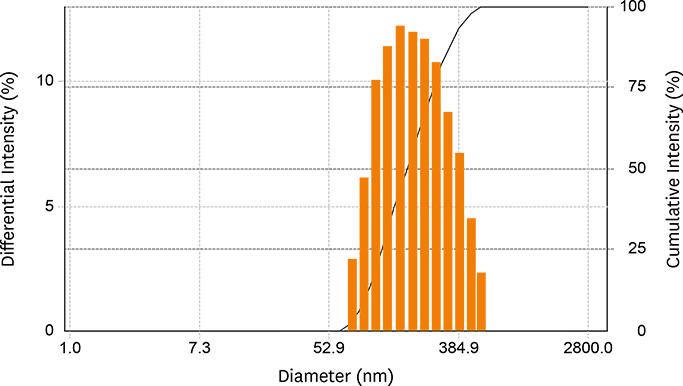
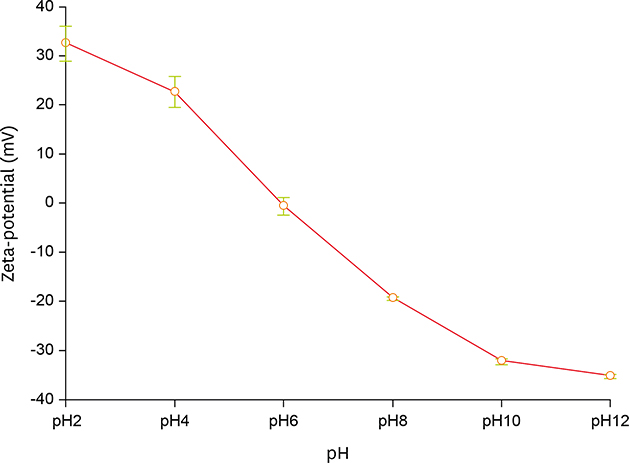
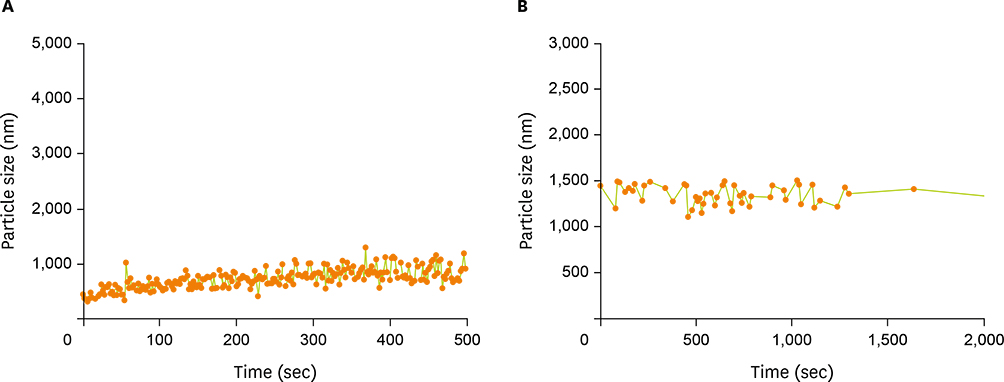
- Acid food indicators can be used as pH indicators for evaluating the quality and freshness of fermented products during the full course of distribution. Iron oxide particles are hardly suspended in water, but partially or completely agglomerated. The agglomeration degree of the iron oxide particles depends on the pH. The pH-dependent particle agglomeration or dispersion can be useful for monitoring the acidity of food. The zeta potential of iron oxide showed a decreasing trend as the pH increased from 2 to 8, while the point of zero charge (PZC) was observed around at pH 6.0-7.0. These results suggested that the size of the iron oxide particles was affected by the change in pH levels. As a result, the particle sizes of iron oxide were smaller at lower pH than at neutral pH. In addition, agglomeration of the iron oxide particles increased as the pH increased from 2 to 7. In the time-dependent aggregation test, the average particle size was 730.4 nm and 1,340.3 nm at pH 2 and 7, respectively. These properties of iron oxide particles can be used to develop an ideal acid indicator for food pH and to monitor food quality, besides a colorant or nutrient for nutrition enhancement and sensory promotion in food industry.
Keyword
MeSH Terms
Figure
Reference
-
1. Fontoin H, Saucier C, Teissedre PL, Glories Y. Effect of pH, ethanol and acidity on astringency and bitterness of grape seed tannin oligomers in model wine solution. Food Qual Prefer. 2008; 19:286–291.
Article2. Wismer-Pedersen J. Quality of pork in relation to rate of pH change post mortem. J Food Sci. 1959; 24:711–727.
Article3. Gibson AM, Bratchell N, Roberts TA. Predicting microbial growth: growth responses of salmonellae in a laboratory medium as affected by pH, sodium chloride and storage temperature. Int J Food Microbiol. 1988; 6:155–178.
Article4. Hong SI, Park WS. Use of color indicators as an active packaging system for evaluating kimchi fermentation. J Food Eng. 2000; 46:67–72.
Article5. Neilands JB. Iron absorption and transport in microorganisms. Annu Rev Nutr. 1981; 1:27–46.
Article6. Mertz W. The essential trace elements. Science. 1981; 213:1332–1338.
Article7. Booth IW, Aukett MA, Logan S. Iron deficiency anaemia in infancy and early childhood. Arch Dis Child. 1997; 76:549–553.8. Neves MC, Pascoal Neto C, Trindade T. Eco-friendly hybrid pigments made of cellulose and iron oxides. J Nanosci Nanotechnol. 2012; 12:6817–6821.
Article9. Shimojo F, Nakano A, Kalia RK, Vashishta P. Electronic processes in fast thermite chemical reactions: a first-principles molecular dynamics study. Phys Rev E Stat Nonlin Soft Matter Phys. 2008; 77:066103.
Article10. Chaudhry Q, Castle L. Food applications of nanotechnologies: an overview of opportunities and challenges for developing countries. Trends Food Sci Technol. 2011; 22:595–603.
Article11. Baalousha M. Aggregation and disaggregation of iron oxide nanoparticles: influence of particle concentration, pH and natural organic matter. Sci Total Environ. 2009; 407:2093–2101.
Article12. Chan T, Verma MS, Gu FX. Optimization of polydiacetylene-coated superparamagnetic magnetite biosensor for colorimetric detection of biomarkers. J Nanosci Nanotechnol. 2015; 15:2628–2633.
Article13. Morillo D, Pérez G, Valiente M. Efficient arsenic(V) and arsenic(III) removal from acidic solutions with Novel Forager Sponge-loaded superparamagnetic iron oxide nanoparticles. J Colloid Interface Sci. 2015; 453:132–141.
Article14. Meng X, Lee K, Kang TY, Ko S. An irreversible ripeness indicator to monitor the CO2 concentration in the headspace of packaged kimchi during storage. Food Sci Biotechnol. 2015; 24:91–97.
Article15. Jung J, Puligundla P, Ko S. Proof-of-concept study of chitosan-based carbon dioxide indicator for food packaging applications. Food Chem. 2012; 135:2170–2174.
Article16. Lee K, Meng X, Kang TY, Ko S. A dye-incorporated chitosan-based CO2 indicator for monitoring of food quality focusing on makgeolli quality during storage. Food Sci Biotechnol. 2015; 24:905–912.
Article17. Jung J, Lee K, Puligundla P, Ko S. Chitosan-based carbon dioxide indicator to communicate the onset of kimchi ripening. Lebensm Wiss Technol. 2013; 54:101–106.
Article18. Hilty FM, Knijnenburg JT, Teleki A, Krumeich F, Hurrell RF, Pratsinis SE, Zimmermann MB. Incorporation of Mg and Ca into nanostructured Fe2O3 improves Fe solubility in dilute acid and sensory characteristics in foods. J Food Sci. 2011; 76:N2–10.
Article19. Gordon T, Perlstein B, Houbara O, Felner I, Banin E, Margel S. Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloids Surf A Physicochem Eng Asp. 2011; 374:1–8.
Article20. Zhang Y, Wang Z, Li X, Wang L, Yin M, Wang L, Chen N, Fan C, Song H. Dietary iron oxide nanoparticles delay aging and ameliorate neurodegeneration in drosophila. Adv Mater. 2016; 28:1387–1393.
Article21. Saleh N, Kim HJ, Phenrat T, Matyjaszewski K, Tilton RD, Lowry GV. Ionic strength and composition affect the mobility of surface-modified Fe0 nanoparticles in water-saturated sand columns. Environ Sci Technol. 2008; 42:3349–3355.
Article22. Navrotsky A, Mazeina L, Majzlan J. Size-driven structural and thermodynamic complexity in iron oxides. Science. 2008; 319:1635–1638.
Article23. Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005; 26:3995–4021.
Article24. Zhang Y, Chen Y, Westerhoff P, Hristovski K, Crittenden JC. Stability of commercial metal oxide nanoparticles in water. Water Res. 2008; 42:2204–2212.
Article25. Domingos RF, Tufenkji N, Wilkinson KI. Aggregation of titanium dioxide nanoparticles: role of a fulvic acid. Environ Sci Technol. 2009; 43:1282–1286.
Article26. French RA, Jacobson AR, Kim B, Isley SL, Penn RL, Baveye PC. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ Sci Technol. 2009; 43:1354–1359.
Article27. Berry CC, Curtis AS. Functionalisation of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys. 2003; 36:R198–206.
Article28. Chomoucka J, Drbohlavova J, Huska D, Adam V, Kizek R, Hubalek J. Magnetic nanoparticles and targeted drug delivering. Pharmacol Res. 2010; 62:144–149.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Iron-enriched Cereal Breakfast and Nutrition Education on the Nutritional Status and Life Style of Elementary School Students
- Quality of Life and Nutritional Outcomes of Billroth I and Billroth II Reconstruction
- A Study on Calcium and Iron Status of Lactating Women
- Nutritional Status after Curative Surgery in Patients with Gastric Cancer: Comparison of Total Versus Subtotal Gastrectomy
- Role of redox iron towards an increase in mortality among patients: a systemic review and meta-analysis