Blood Res.
2019 Jun;54(2):87-101. 10.5045/br.2019.54.2.87.
Role of redox iron towards an increase in mortality among patients: a systemic review and meta-analysis
- Affiliations
-
- 1Department of Transfusion Medicine and Blood Bank, All India Institute of Medical Sciences Raipur, Chhattisgarh, India. sunray2077@gmail.com
- KMID: 2451008
- DOI: http://doi.org/10.5045/br.2019.54.2.87
Abstract
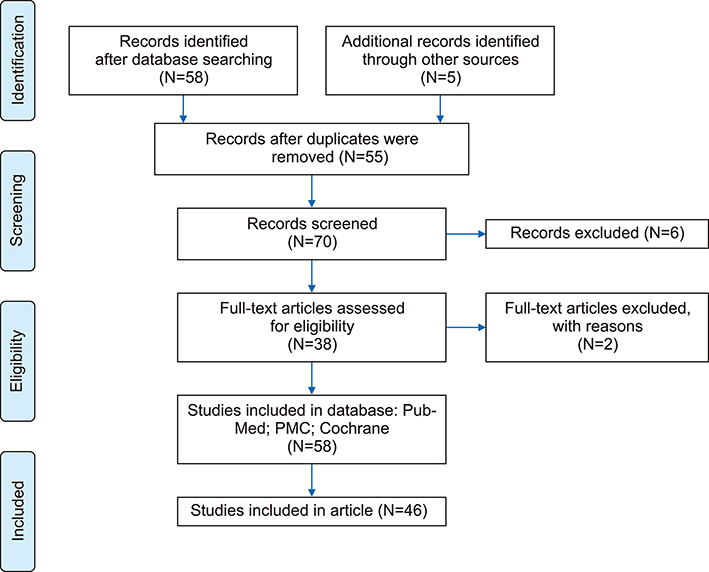
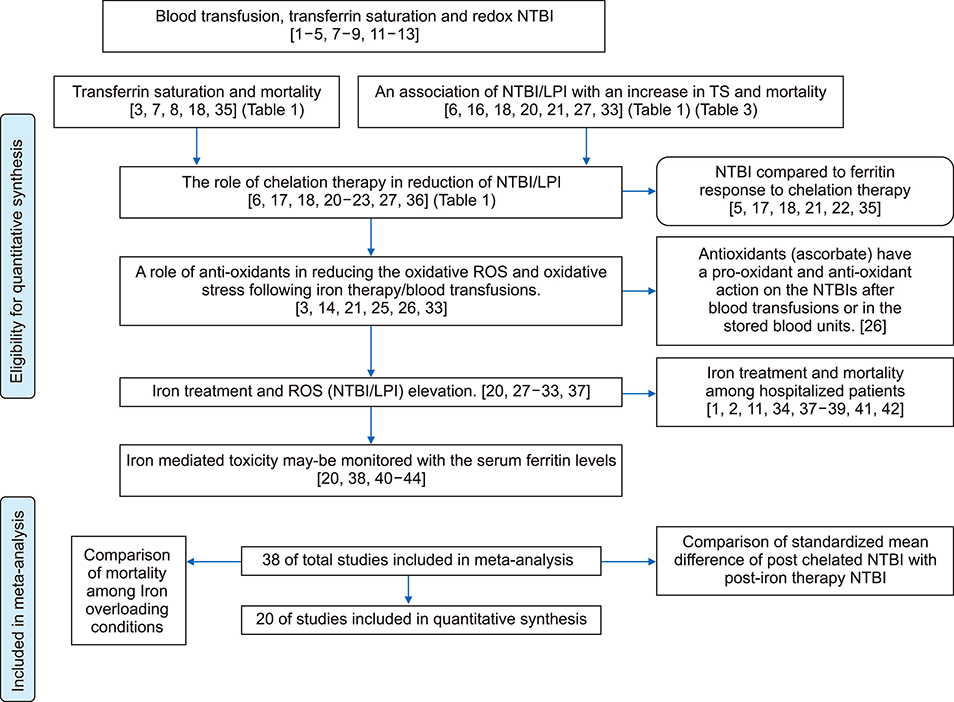
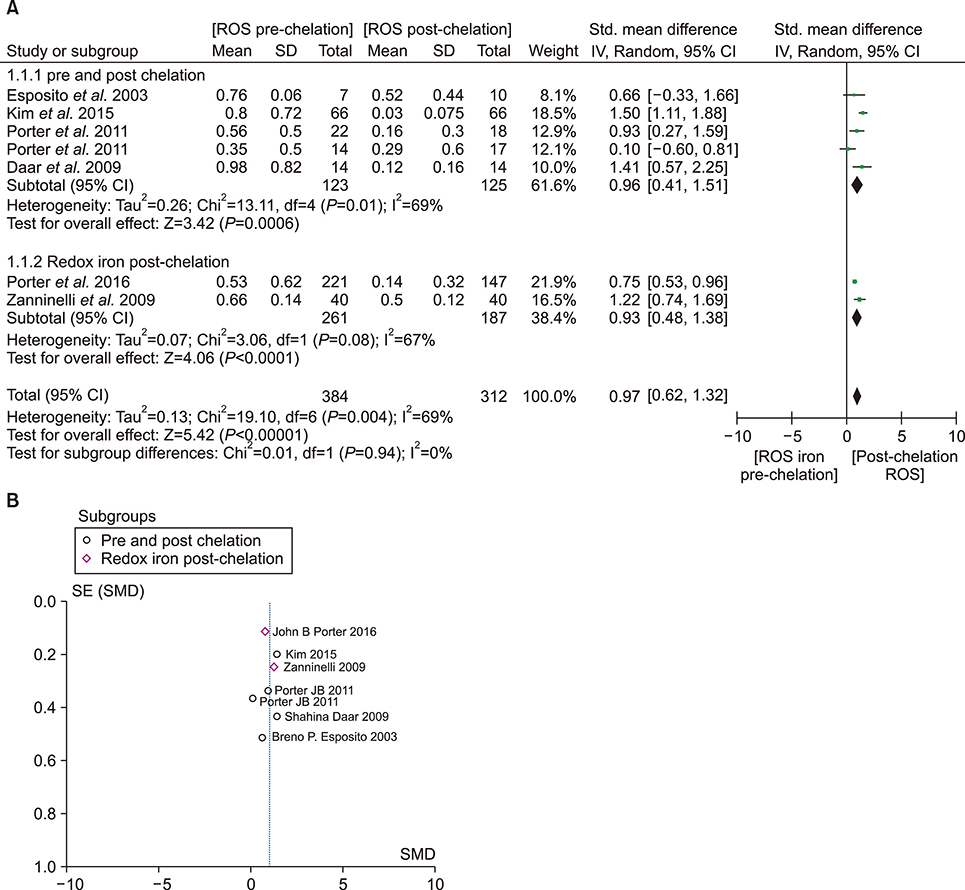
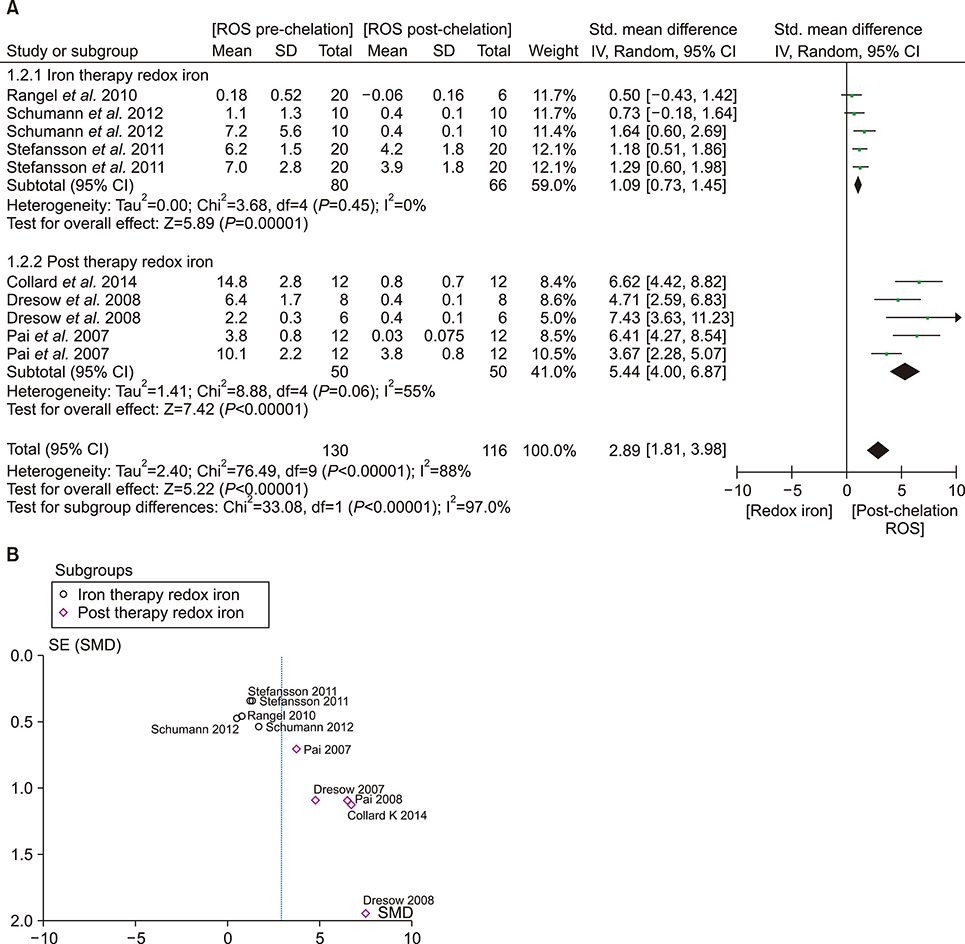
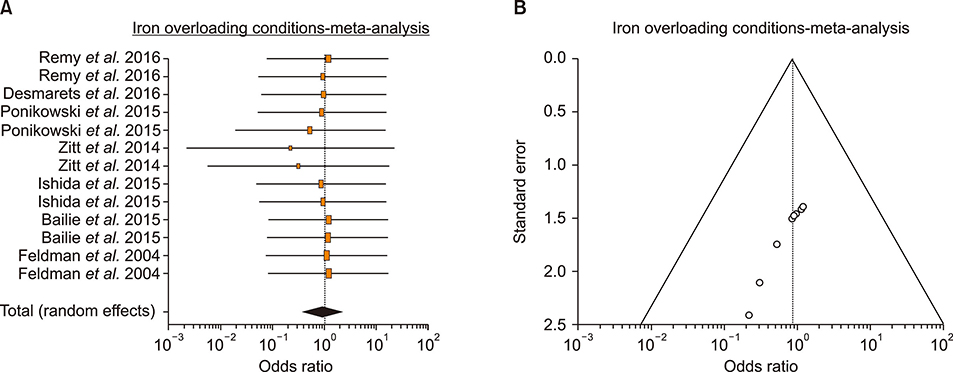
- An increase in biochemical concentrations of non-transferrin bound iron (NTBI) within the patients with an increase in serum iron concentration was evaluated with the following objectives: (a) Iron overloading diseases/conditions with free radicle form of "˜iron containing' reactive oxygen species (ROS) and its imbalance mediated mortality, and (b) Intervention with iron containing drugs in context to increased redox iron concentration and treatment induced mortality. Literature search was done within Pubmed and cochrane review articles. The Redox iron levels are increased during dys-erythropoiesis and among transfusion recipient population and are responsive to iron-chelation therapy. Near expiry "˜stored blood units' show a significant rise in the ROS level. Iron mediated ROS damage may be estimated by the serum antioxidant level, and show reduction in toxicity with high antioxidant, low pro-oxidant levels. Iron drug therapy causes a significant increase in NTBI and labile iron levels. Hospitalized patients on iron therapy however show a lower mortality rate. Serum ferritin is a mortality indicator among the high-dose iron therapy and transfusion dependent population. The cumulative difference of pre-chelation to post chelation ROS iron level was 0.97 (0.62; 1.32; N=261) among the transfusion dependent subjects and 2.89 (1.81-3.98; N=130) in the post iron therapy "˜iron ROS' group. In conclusion, iron mediated mortality may not be mediated by redox iron among multi-transfused and iron overloaded patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Remy KE, Sun J, Wang D, et al. Transfusion of recently donated (fresh) red blood cells (RBCs) does not improve survival in comparison with current practice, while safety of the oldest stored units is yet to be established: a meta-analysis. Vox Sang. 2016; 111:43–54.
Article2. Brunskill SJ, Wilkinson KL, Doree C, Trivella M, Stanworth S. Transfusion of fresher versus older red blood cells for all conditions. Cochrane Database Syst Rev. 2015; CD010801.
Article3. Collard K, White D, Copplestone A. The influence of storage age on iron status, oxidative stress and antioxidant protection in paediatric packed cell units. Blood Transfus. 2014; 12:210–219.4. Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011; 118:6675–6682.
Article5. Hirano K, Morinobu T, Kim H, et al. Blood transfusion increases radical promoting non-transferrin bound iron in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2001; 84:F188–F193.
Article6. Aydinok Y, Evans P, Manz CY, Porter JB. Timed non-transferrin bound iron determinations probe the origin of chelatable iron pools during deferiprone regimens and predict chelation response. Haematologica. 2012; 97:835–841.
Article7. Ellervik C, Tybjærg-Hansen A, Nordestgaard BG. Total mortality by transferrin saturation levels: two general population studies and a metaanalysis. Clin Chem. 2011; 57:459–466.
Article8. Chua AC, Knuiman MW, Trinder D, Divitini ML, Olynyk JK. Higher concentrations of serum iron and transferrin saturation but not serum ferritin are associated with cancer outcomes. Am J Clin Nutr. 2016; 104:736–742.
Article9. Schaer DJ, Buehler PW. Cell-free hemoglobin and its scavenger proteins: new disease models leading the way to targeted therapies. Cold Spring Harb Perspect Med. 2013; 3.
Article10. Gladwin MT, Kanias T, Kim-Shapiro DB. Hemolysis and cell-free hemoglobin drive an intrinsic mechanism for human disease. J Clin Invest. 2012; 122:1205–1208.
Article11. Desmarets M, Bardiaux L, Benzenine E, et al. Effect of storage time and donor sex of transfused red blood cells on 1-year survival in patients undergoing cardiac surgery: an observational study. Transfusion. 2016; 56:1213–1222.
Article12. Solomon SB, Wang D, Sun J, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013; 121:1663–1672.
Article13. Cortes-Puch I, Remy KE, Solomon SB, et al. In a canine pneumonia model of exchange transfusion, altering the age but not the volume of older red blood cells alters outcome. Transfusion. 2015; 55:2564–2575.
Article14. Nagababu E, Scott AV, Johnson DJ, et al. Oxidative stress and rheologic properties of stored red blood cells before and after transfusion to surgical patients. Transfusion. 2016; 56:1101–1111.
Article15. Blanchette NL, Manz DH, Torti FM, Torti SV. Modulation of hepcidin to treat iron deregulation: potential clinical applications. Expert Rev Hematol. 2016; 9:169–186.
Article16. Ginzburg Y, Rivella S. β-thalassemia: a model for elucidating the dynamic regulation of ineffective erythropoiesis and iron metabolism. Blood. 2011; 118:4321–4330.
Article17. Porter JB, Lin KH, Beris P, et al. Response of iron overload to deferasirox in rare transfusion-dependent anaemias: equivalent effects on serum ferritin and labile plasma iron for haemolytic or production anaemias. Eur J Haematol. 2011; 87:338–348.
Article18. Pootrakul P, Breuer W, Sametband M, Sirankapracha P, Hershko C, Cabantchik ZI. Labile plasma iron (LPI) as an indicator of chelatable plasma redox activity in iron-overloaded beta-thalassemia/HbE patients treated with an oral chelator. Blood. 2004; 104:1504–1510.
Article19. Miura K, Taura K, Kodama Y, Schnabl B, Brenner DA. Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity. Hepatology. 2008; 48:1420–1429.
Article20. Malyszko J, Koc-Zorawska E, Levin-Iaina N, et al. Iron metabolism in hemodialyzed patients - a story half told? Arch Med Sci. 2014; 10:1117–1122.21. Esposito BP, Breuer W, Sirankapracha P, Pootrakul P, Hershko C, Cabantchik ZI. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood. 2003; 102:2670–2677.
Article22. Daar S, Pathare A, Nick H, et al. Reduction in labile plasma iron during treatment with deferasirox, a once-daily oral iron chelator, in heavily iron-overloaded patients with beta-thalassaemia. Eur J Haematol. 2009; 82:454–457.
Article23. Kim IH, Moon JH, Lim SN, et al. Efficacy and safety of deferasirox estimated by serum ferritin and labile plasma iron levels in patients with aplastic anemia, myelodysplastic syndrome, or acute myeloid leukemia with transfusional iron overload. Transfusion. 2015; 55:1613–1620.
Article24. Cappellini MD, Porter J, El-Beshlawy A, et al. Tailoring iron chelation by iron intake and serum ferritin: the prospective EPIC study of deferasirox in 1744 patients with transfusion-dependent anemias. Haematologica. 2010; 95:557–566.
Article25. Breuer W, Ghoti H, Shattat A, et al. Non-transferrin bound iron in thalassemia: differential detection of redox active forms in children and older patients. Am J Hematol. 2012; 87:55–61.
Article26. Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta. 2012; 1826:443–457.
Article27. Geisser P, Burckhardt S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics. 2011; 3:12–33.
Article28. Schumann K, Solomons NW, Romero-Abal ME, Orozco M, Weiss G, Marx J. Oral administration of ferrous sulfate, but not of iron polymaltose or sodium iron ethylenediaminetetraacetic acid (NaFeEDTA), results in a substantial increase of non-transferrin-bound iron in healthy iron-adequate men. Food Nutr Bull. 2012; 33:128–136.
Article29. Stefansson BV, Haraldsson B, Nilsson U. Acute oxidative stress following intravenous iron injection in patients on chronic hemodialysis: a comparison of iron-sucrose and iron-dextran. Nephron Clin Pract. 2011; 118:c249–c256.
Article30. Pai AB, Boyd AV, McQuade CR, Harford A, Norenberg JP, Zager PG. Comparison of oxidative stress markers after intravenous administration of iron dextran, sodium ferric gluconate, and iron sucrose in patients undergoing hemodi-alysis. Pharmacotherapy. 2007; 27:343–350.
Article31. Dresow B, Petersen D, Fischer R, Nielsen P. Non-transferrin-bound iron in plasma following administration of oral iron drugs. Biometals. 2008; 21:273–276.
Article32. Esposito BP, Breuer W, Slotki I, Cabantchik ZI. Labile iron in parenteral iron formulations and its potential for generating plasma nontransferrin-bound iron in dialysis patients. Eur J Clin Invest. 2002; 32:Suppl 1. 42–49.
Article33. Agarwal R, Vasavada N, Sachs NG, Chase S. Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int. 2004; 65:2279–2289.
Article34. Vitrano A, Calvaruso G, Lai E, et al. The era of comparable life expectancy between thalassaemia major and intermedia: Is it time to revisit the major-intermedia dichotomy? Br J Haematol. 2017; 176:124–130.
Article35. Porter JB, El-Alfy M, Viprakasit V, et al. Utility of labile plasma iron and transferrin saturation in addition to serum ferritin as iron overload markers in different underlying anemias before and after deferasirox treatment. Eur J Haematol. 2016; 96:19–26.
Article36. Zanninelli G, Breuer W, Cabantchik ZI. Daily labile plasma iron as an indicator of chelator activity in thalassaemia major patients. Br J Haematol. 2009; 147:744–751.
Article37. Rangel EB, Esposito BP, Carneiro FD, et al. Labile plasma iron generation after intravenous iron is time-dependent and transitory in patients undergoing chronic hemodialysis. Ther Apher Dial. 2010; 14:186–192.
Article38. Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J. 2015; 36:657–668.
Article39. Zitt E, Sturm G, Kronenberg F, et al. Iron supplementation and mortality in incident dialysis patients: an observa-tional study. PLoS One. 2014; 9:e114144.
Article40. Ishida JH, Marafino BJ, McCulloch CE, et al. Receipt of intravenous iron and clinical outcomes among hemodialysis patients hospitalized for infection. Clin J Am Soc Nephrol. 2015; 10:1799–1805.
Article41. Bailie GR, Larkina M, Goodkin DA, et al. Data from the Dialysis Outcomes and Practice Patterns Study validate an association between high intravenous iron doses and mortality. Kidney Int. 2015; 87:162–168.
Article42. Feldman HI, Joffe M, Robinson B, et al. Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc Nephrol. 2004; 15:1623–1632.
Article43. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. 2012; 2:Suppl. 279–335.44. Ribeiro S, Belo L, Reis F, Santos-Silva A. Iron therapy in chronic kidney disease: Recent changes, benefits and risks. Blood Rev. 2016; 30:65–72.
Article45. Cladellas M, Farre N, Comín-Colet J, et al. Effects of preoperative intravenous erythropoietin plus iron on outcome in anemic patients after cardiac valve replacement. Am J Cardiol. 2012; 110:1021–1026.
Article46. Hoffbrand AV, Higgs DG, Keeling DM, Mehta AB, editors. Postgraduate haematology. 7th ed. Oxford, UK: Wiley-Blackwell;2016. p. 20–52.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Helicobacter pylori and Hematologic Diseases
- Basic Understanding of Iron Metabolism
- The Effect of Polymyxin B-Immobilized Fiber Column Hemoperfusion for Sepsis: A Systemic Review and Meta-Analysis
- An Introduction of the Systematic Review and Meta-Analysis
- Weekend Admission and Mortality in Patients With Traumatic Brain Injury: A Meta-analysis