J Vet Sci.
2015 Sep;16(3):253-263. 10.4142/jvs.2015.16.3.253.
Comparative proteomic analysis of proteins expression changes in the mammary tissue of cows infected with Escherichia coli mastitis
- Affiliations
-
- 1Institute of Animal Science and Veterinary Medicine, Anhui Academy of Agricultural Sciences, Hefei 230031, China. yyongxin@yahoo.com
- KMID: 2344297
- DOI: http://doi.org/10.4142/jvs.2015.16.3.253
Abstract
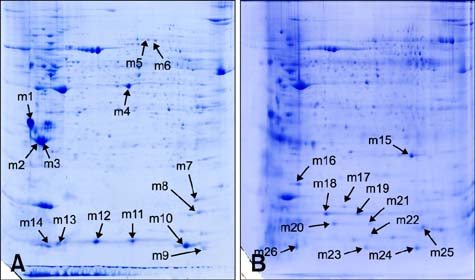
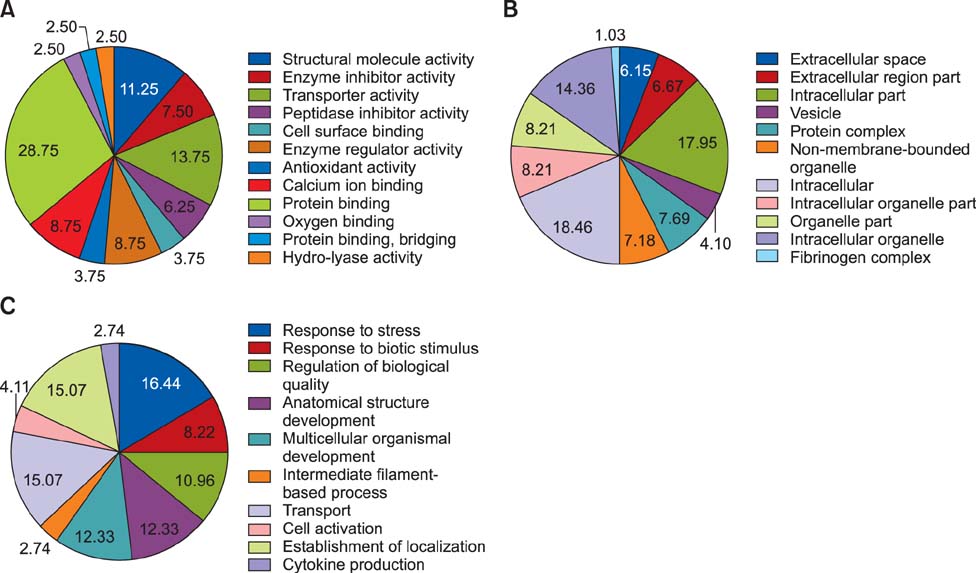
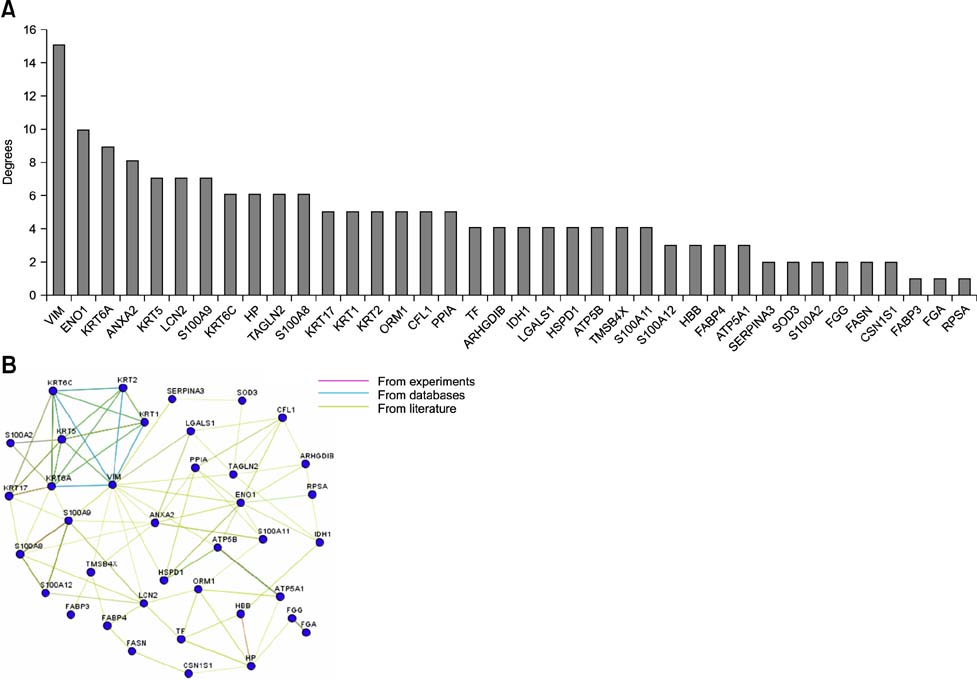
- Cows infected with Escherichia (E.) coli usually experience severe clinical symptoms, including damage to mammary tissues, reduced milk yield, and altered milk composition. In order to investigate the host response to E. coli infection and discover novel markers for mastitis treatment, mammary tissue samples were collected from healthy cows and bovines with naturally occurring severe E. coli mastitis. Changes of mammary tissue proteins were examined using two-dimensional gel electrophoresis and label-free proteomic approaches. A total of 95 differentially expressed proteins were identified. Of these, 56 proteins were categorized according to molecular function, cellular component, and biological processes. The most frequent biological processes influenced by the proteins were response to stress, transport, and establishment of localization. Furthermore, a network analysis of the proteins with altered expression in mammary tissues demonstrated that these factors are predominantly involved with binding and structural molecule activities. Vimentin and alpha-enolase were central "functional hubs" in the network. Based on results from the present study, disease-induced alterations of protein expression in mammary glands and potential markers for the effective treatment of E. coli mastitis were identified. These data have also helped elucidate defense mechanisms that protect the mammary glands and promote the pathogenesis of E. coli mastitis.
Keyword
MeSH Terms
-
Animals
Cattle
Electrophoresis, Gel, Two-Dimensional/veterinary
Escherichia coli/*physiology
Escherichia coli Infections/genetics/immunology/microbiology/*veterinary
Female
Mammary Glands, Animal/*immunology/pathology
Mastitis, Bovine/*genetics/immunology/microbiology
Proteome/*genetics/metabolism
*Proteomics
Proteome
Figure
Reference
-
1. Alonso-Fauste I, Andrés M, Iturralde M, Lampreave F, Gallart J, Alava MA. Proteomic characterization by 2-DE in bovine serum and whey from healthy and mastitis affected farm animals. J Proteomics. 2012; 75:3015–3030.
Article2. Anaya-López JL, Contreras-Guzmán OE, Cárabez-Trejo A, Baizabal-Aguirre VM, López-Meza JE, Valdez-Alarcón JJ, Ochoa-Zarzosa A. Invasive potential of bacterial isolates associated with subclinical bovine mastitis. Res Vet Sci. 2006; 81:358–361.
Article3. Bannerman DD, Kauf AC, Paape MJ, Springer HR, Goff JP. Comparison of Holstein and Jersey innate immune responses to Escherichia coli intramammary infection. J Dairy Sci. 2008; 91:2225–2235.
Article4. Boehmer JL. Proteomic analyses of host and pathogen responses during bovine mastitis. J Mammary Gland Biol Neoplasia. 2011; 16:323–338.
Article5. Boehmer JL, Bannerman DD, Shefcheck K, Ward JL. Proteomic analysis of differentially expressed proteins in bovine milk during experimentally induced Escherichia coli mastitis. J Dairy Sci. 2008; 91:4206–4218.
Article6. Boehmer JL, Ward JL, Peters RR, Shefcheck KJ, McFarland MA, Bannerman DD. Proteomic analysis of the temporal expression of bovine milk proteins during coliform mastitis and label-free relative quantification. J Dairy Sci. 2010; 93:593–603.
Article7. Buitenhuis B, Røntved CM, Edwards SM, Ingvartsen KL, Sørensen P. In depth analysis of genes and pathways of the mammary gland involved in the pathogenesis of bovine Escherichia coli-mastitis. BMC Genomics. 2011; 12:130.8. Burvenich C, Van Merris V, Mehrzad J, Diez-Fraile A, Duchateau L. Severity of E. coli mastitis is mainly determined by cow factors. Vet Res. 2003; 34:521–564.9. Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004; 25:1327–1333.
Article10. Fink-Retter A, Czerwenka K, Gschwantler-Kaulich D, Hudelist G, Pischinger K, Manavi M, Kubista E, Singer CF. Proteomics in mammary cancer research. Eur J Gynaecol Oncol. 2009; 30:635–639.
Article11. Goldstein AL, Hannappel E, Sosne G, Kleinman HK. Thymosin β4: a multi-functional regenerative peptide. Basic properties and clinical applications. Expert Opin Biol Ther. 2012; 12:37–51.
Article12. Hiss S, Mielenz M, Bruckmaier RM, Sauerwein H. Haptoglobin concentrations in blood and milk after endotoxin challenge and quantification of mammary Hp mRNA expression. J Dairy Sci. 2004; 87:3778–3784.
Article13. Hogarth CJ, Fitzpatrick JL, Nolan AM, Young FJ, Pitt A, Eckersall PD. Differential protein composition of bovine whey: a comparison of whey from healthy animals and from those with clinical mastitis. Proteomics. 2004; 4:2094–2100.
Article14. Hooper SD, Bork P. Medusa: a simple tool for interaction graph analysis. Bioinformatics. 2005; 21:4432–4433.
Article15. Jensen K, Günther J, Talbot R, Petzl W, Zerbe H, Schuberth HJ, Seyfert HM, Glass EJ. Escherichia coli- and Staphylococcus aureus-induced mastitis differentially modulate transcriptional responses in neighbouring uninfected bovine mammary gland quarters. BMC Genomics. 2013; 14:36.
Article16. Lippolis JD, Reinhardt TA. Proteomic survey of bovine neutrophils. Vet Immunol Immunopathol. 2005; 103:53–65.
Article17. Lutzow YCS, Donaldson L, Gray CP, Vuocolo T, Pearson RD, Reverter A, Byrne KA, Sheehy PA, Windon R, Tellam RL. Identification of immune genes and proteins involved in the response of bovine mammary tissue to Staphylococcus aureus infection. BMC Vet Res. 2008; 4:18.18. Mannherz HG, Hannappel E. The β-thymosins: intracellular and extracellular activities of a versatile actin binding protein family. Cell Motil Cytoskeleton. 2009; 66:839–851.
Article19. McLean J, Liu HN, Miletic D, Weng YC, Rogaeva E, Zinman L, Kriz J, Robertson J. Distinct biochemical signatures characterize peripherin isoform expression in both traumatic neuronal injury and motor neuron disease. J Neurochem. 2010; 114:1177–1192.
Article20. Old WM, Meyer-Arendt K, Aveline-Wolf L, Pierce KG, Mendoza A, Sevinsky JR, Resing KA, Ahn NG. Comparison of label-free methods for quantifying human proteins by shotgun proteomics. Mol Cell Proteomics. 2005; 4:1487–1502.
Article21. Perera C, McNeil HP, Geczy CL. S100 Calgranulins in inflammatory arthritis. Immunol Cell Biol. 2010; 88:41–49.
Article22. Pham TV, Piersma SR, Warmoes M, Jimenez CR. On the beta-binomial model for analysis of spectral count data in label-free tandem mass spectrometry-based proteomics. Bioinformatics. 2010; 26:363–369.
Article23. Philp D, Kleinman HK. Animal studies with thymosin β4, a multifunctional tissue repair and regeneration peptide. Ann N Y Acad Sci. 2010; 1194:81–86.
Article24. Qiu P, Wheater MK, Qiu Y, Sosne G. Thymosin β4 inhibits TNF-α-induced NF-κB activation, IL-8 expression, and the sensitizing effects by its partners PINCH-1 and ILK. FASEB J. 2011; 25:1815–1826.
Article25. Rawson P, Stockum C, Peng L, Manivannan B, Lehnert K, Ward HE, Berry SD, Davis SR, Snell RG, McLauchlan D, Jordan TW. Metabolic proteomics of the liver and mammary gland during lactation. J Proteomics. 2012; 75:4429–4435.
Article26. Regenhard P, Petzl W, Zerbe H, Sauerwein H. The antibacterial psoriasin is induced by E. coli infection in the bovine udder. Vet Microbiol. 2010; 143:293–298.
Article27. Reinhardt TA, Sacco RE, Nonnecke BJ, Lippolis JD. Bovine milk proteome: quantitative changes in normal milk exosomes, milk fat globule membranes and whey proteomes resulting from Staphylococcus aureus mastitis. J Proteomics. 2013; 82:141–154.
Article28. Ryu YK, Lee YS, Lee GH, Song KS, Kim YS, Moon EY. Regulation of glycogen synthase kinase-3 by thymosin beta-4 is associated with gastric cancer cell migration. Int J Cancer. 2012; 131:2067–2077.
Article29. Sears PM, González RN, Wilson DJ, Han HR. Procedures for mastitis diagnosis and control. Vet Clin North Am Food Anim Pract. 1993; 9:445–468.
Article30. Smolenski GA, Wieliczko RJ, Pryor SM, Broadhurst MK, Wheeler TT, Haigh BJ. The abundance of milk cathelicidin proteins during bovine mastitis. Vet Immunol Immunopathol. 2011; 143:125–130.
Article31. Suojala L, Orro T, Järvinen H, Saatsi J, Pyörälä S. Acute phase response in two consecutive experimentally induced E. coli intramammary infections in dairy cows. Acta Vet Scand. 2008; 50:18.32. Toivola D, Strnad P, Habtezion A, Omary M. Intermediate filaments take the heat as stress proteins. Trends Cell Biol. 2010; 20:79–91.
Article33. Urech E, Puhan Z, Schällibaum M. Changes in milk protein fraction as affected by subclinical mastitis. J Dairy Sci. 1999; 82:2402–2411.
Article34. Vanselow J, Yang W, Herrmann J, Zerbe H, Schuberth HJ, Petzl W, Tomek W, Seyfert HM. DNA-remethylation around a STAT5-binding enhancer in the αS1-casein promoter is associated with abrupt shutdown of αS1-casein synthesis during acute mastitis. J Mol Endocrinol. 2006; 37:463–477.
Article35. Warmoes M, Jaspers JE, Pham TV, Piersma SR, Oudgenoeg G, Massink MP, Waisfisz Q, Rottenberg S, Boven E, Jonkers J, Jimenez CR. Proteomics of mouse BRCA1-deficient mammary tumors identifies DNA repair proteins withcancer. Mol Cell Proteomics. 2012; 11:M111.013334.36. Xiao S, Tjostheim S, Sanelli T, McLean JR, Horne P, Fan Y, Ravits J, Strong MJ, Robertson J. An aggregate-inducing peripherin isoform generated through intron retention is upregulated in amyotrophic lateral sclerosis and associated with disease pathology. J Neurosci. 2008; 28:1833–1840.
Article37. Yang Y, Zhao X, Zhang Y. Proteomic analysis of mammary tissues from healthy cows and clinical mastitic cows for identification of disease-related proteins. Vet Res Commun. 2009; 33:295–303.
Article38. Yu C, Shi ZR, Chu CY, Lee KH, Zhao X, Lee JW. Expression of bovine granulocyte chemotactic protein-2 (GCP-2) in neutrophils and a mammary epithelial cell line (MAC-T) in response to various bacterial cell wall components. Vet J. 2010; 186:89–95.
Article39. Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004; 75:39–48.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of the California mastitis test usage in smallholder dairy herds and risk of violative antimicrobial residues
- Therapeutic effect of oregano essential oil on subclinical bovine mastitis caused by Staphylococcus aureus and Escherichia coli
- Successful treatment of recurrent subclinical mastitis in cows caused by enrofloxacin resistant bacteria by means of the sequential intramammary infusion of enrofloxacin HCl-2H 2 O and ceftiofur HCl: a clinical trial
- Expression of human CTL?4 extracellular domain in escherichia coli
- Innate immune response of bovine mammary epithelial cells to Mycoplasma bovis