Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part I. General Guidance and Tips
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul 05505, Korea. parksh.radiology@gmail.com
- 2Department of Biostatistics, Korea University College of Medicine, Seoul 02841, Korea.
- KMID: 2344271
- DOI: http://doi.org/10.3348/kjr.2015.16.6.1175
Abstract
- In the field of diagnostic test accuracy (DTA), the use of systematic review and meta-analyses is steadily increasing. By means of objective evaluation of all available primary studies, these two processes generate an evidence-based systematic summary regarding a specific research topic. The methodology for systematic review and meta-analysis in DTA studies differs from that in therapeutic/interventional studies, and its content is still evolving. Here we review the overall process from a practical standpoint, which may serve as a reference for those who implement these methods.
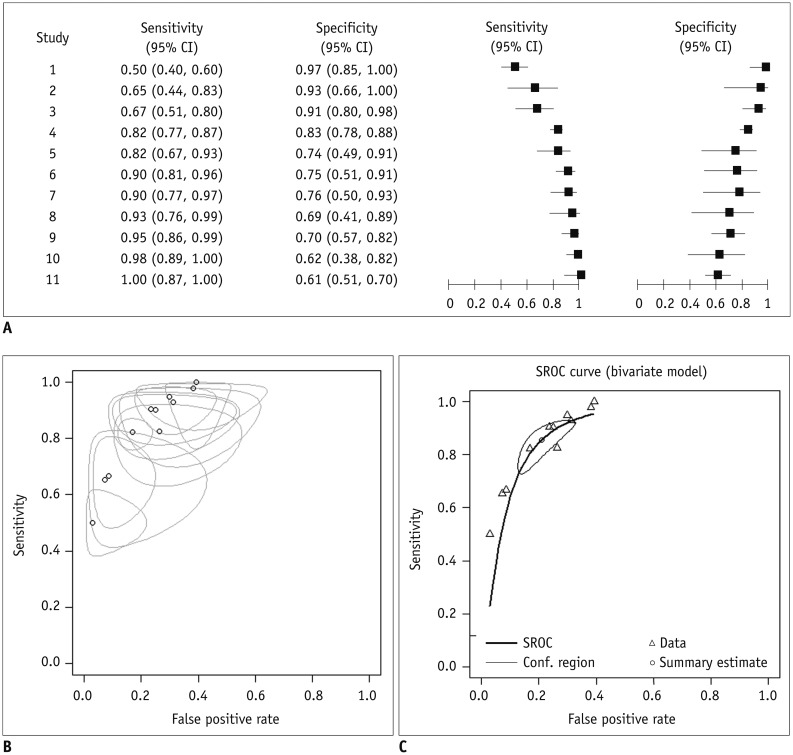
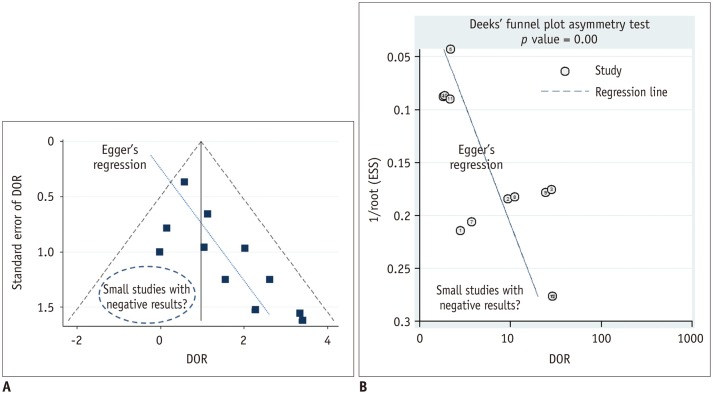
Figure
Cited by 3 articles
-
Technical Performance of Two-Dimensional Shear Wave Elastography for Measuring Liver Stiffness: A Systematic Review and Meta-Analysis
Dong Wook Kim, Chong Hyun Suh, Kyung Won Kim, Junhee Pyo, Chan Park, Seung Chai Jung
Korean J Radiol. 2019;20(6):880-893. doi: 10.3348/kjr.2018.0812.Cardiac CT for Measurement of Right Ventricular Volume and Function in Comparison with Cardiac MRI: A Meta-Analysis
Jin Young Kim, Young Joo Suh, Kyunghwa Han, Young Jin Kim, Byoung Wook Choi
Korean J Radiol. 2020;21(4):450-461. doi: 10.3348/kjr.2019.0499.Diagnostic Performance of Ultrasound-Based Risk Stratification Systems for Thyroid Nodules: A Systematic Review and Meta-Analysis
Leehi Joo, Min Kyoung Lee, Ji Ye Lee, Eun Ju Ha, Dong Gyu Na
Endocrinol Metab. 2023;38(1):117-128. doi: 10.3803/EnM.2023.1670.
Reference
-
1. Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and Presenting Results. In : Deeks JJ, Bossuyt PM, Gatsonis C, editors. The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. 2010. Available from: http://srdta.cochrane.org/.2. Tunis AS, McInnes MD, Hanna R, Esmail K. Association of study quality with completeness of reporting: have completeness of reporting and quality of systematic reviews and meta-analyses in major radiology journals changed since publication of the PRISMA statement? Radiology. 2013; 269:413–426. PMID: 23824992.
Article3. Irwig L, Tosteson AN, Gatsonis C, Lau J, Colditz G, Chalmers TC, et al. Guidelines for meta-analyses evaluating diagnostic tests. Ann Intern Med. 1994; 120:667–676. PMID: 8135452.
Article4. Trikalinos TA, Balion CM, Coleman CI, Griffith L, Santaguida PL, Vandermeer B, et al. Chapter 8: meta-analysis of test performance when there is a "gold standard". J Gen Intern Med. 2012; 27(Suppl 1):S56–S66. PMID: 22648676.
Article5. Lee J, Kim KW, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers--part ii. statistical methods of meta-analysis. Korean J Radiol. 2015; 16:1188–1196.
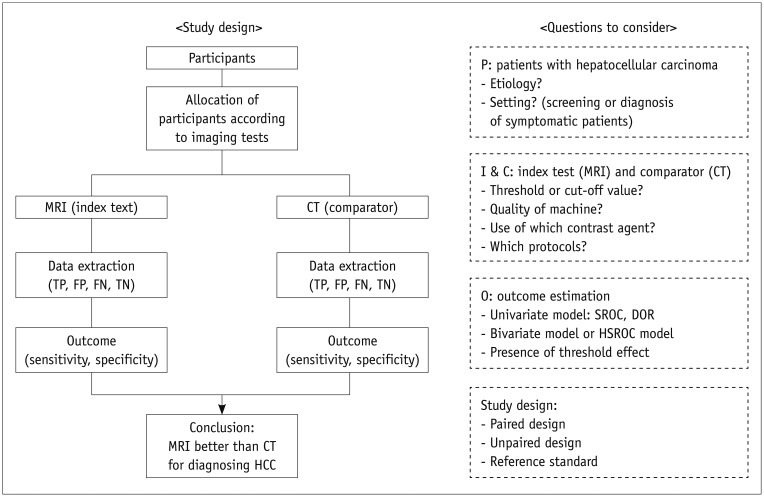
Article6. Jaeschke R, Guyatt GH, Sackett DL. The Evidence-Based Medicine Working Group. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? JAMA. 1994; 271:703–707. PMID: 8309035.7. Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015; 275:97–109. PMID: 25559230.
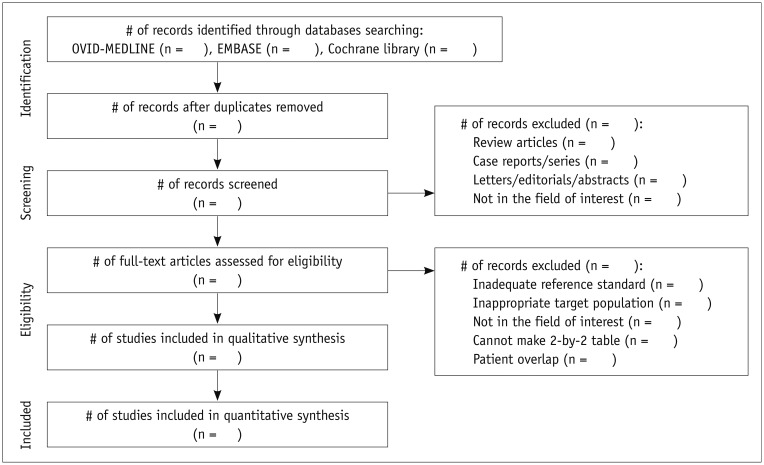
Article8. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015; 4:1. PMID: 25554246.
Article9. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007; 7:10. PMID: 17302989.
Article10. Sampson M, Barrowman NJ, Moher D, Klassen TP, Pham B, Platt R, et al. Should meta-analysts search Embase in addition to Medline? J Clin Epidemiol. 2003; 56:943–955. PMID: 14568625.
Article11. Staunton M. Evidence-based radiology: steps 1 and 2--asking answerable questions and searching for evidence. Radiology. 2007; 242:23–31. PMID: 17185659.
Article12. Moher D, Liberati A, Tetzlaff J, Altman DG. the PRISMA statement. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–269. W64. PMID: 19622511.
Article13. Jones CM, Ashrafian H, Darzi A, Athanasiou T. Guidelines for diagnostic tests and diagnostic accuracy in surgical research. J Invest Surg. 2010; 23:57–65. PMID: 20233006.
Article14. Whiting P, Rutjes AW, Reitsma JB, Glas AS, Bossuyt PM, Kleijnen J. Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Ann Intern Med. 2004; 140:189–202. PMID: 14757617.15. Dodd JD. Evidence-based practice in radiology: steps 3 and 4--appraise and apply diagnostic radiology literature. Radiology. 2007; 242:342–354. PMID: 17255406.
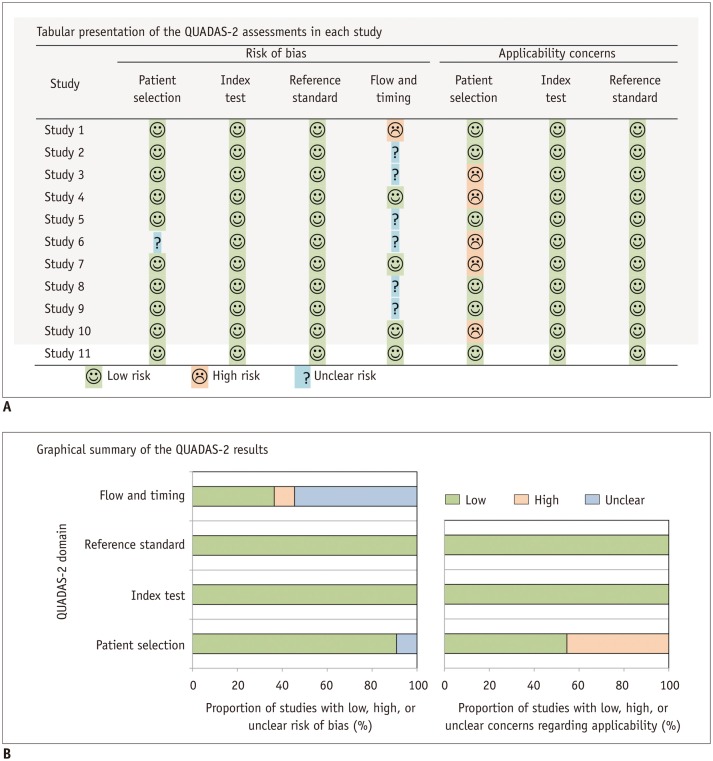
Article18. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003; 3:25. PMID: 14606960.
Article19. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155:529–536. PMID: 22007046.
Article20. Wade R, Corbett M, Eastwood A. Quality assessment of comparative diagnostic accuracy studies: our experience using a modified version of the QUADAS-2 tool. Res Synth Methods. 2013; 4:280–286. PMID: 26053845.
Article21. Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Cochrane Diagnostic Test Accuracy Working Group. Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008; 149:889–897. PMID: 19075208.
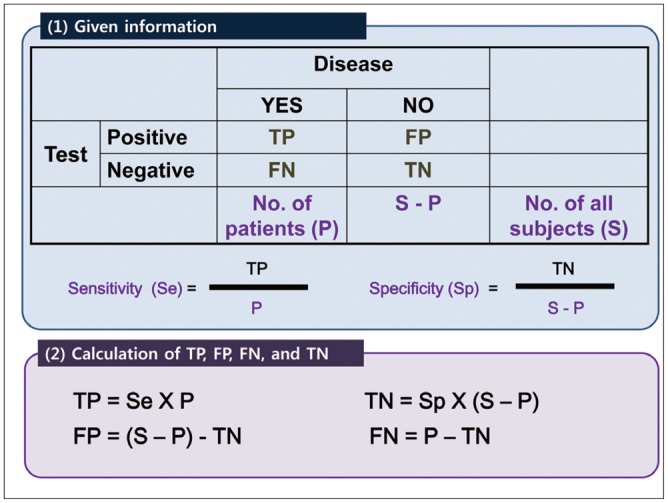
Article22. Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005; 58:982–990. PMID: 16168343.
Article23. Halligan S, Altman DG. Evidence-based practice in radiology: steps 3 and 4--appraise and apply systematic reviews and meta-analyses. Radiology. 2007; 243:13–27. PMID: 17392245.
Article24. Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001; 323:157–162. PMID: 11463691.
Article25. Devillé WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, van der Windt DA, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002; 2:9. PMID: 12097142.
Article26. Chappell FM, Raab GM, Wardlaw JM. When are summary ROC curves appropriate for diagnostic meta-analyses? Stat Med. 2009; 28:2653–2668. PMID: 19591118.
Article27. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560. PMID: 12958120.
Article28. Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and related biases. Health Technol Assess. 2000; 4:1–115.
Article29. Tatsioni A, Zarin DA, Aronson N, Samson DJ, Flamm CR, Schmid C, et al. Challenges in systematic reviews of diagnostic technologies. Ann Intern Med. 2005; 142(12 Pt 2):1048–1055. PMID: 15968029.
Article30. Mulherin SA, Miller WC. Spectrum bias or spectrum effect? Subgroup variation in diagnostic test evaluation. Ann Intern Med. 2002; 137:598–602. PMID: 12353947.
Article31. de Groot JA, Dendukuri N, Janssen KJ, Reitsma JB, Brophy J, Joseph L, et al. Adjusting for partial verification or workup bias in meta-analyses of diagnostic accuracy studies. Am J Epidemiol. 2012; 175:847–853. PMID: 22422923.
Article32. Leeflang MM, Moons KG, Reitsma JB, Zwinderman AH. Bias in sensitivity and specificity caused by data-driven selection of optimal cutoff values: mechanisms, magnitude, and solutions. Clin Chem. 2008; 54:729–737. PMID: 18258670.
Article33. Ewald B. Post hoc choice of cut points introduced bias to diagnostic research. J Clin Epidemiol. 2006; 59:798–801. PMID: 16828672.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part II. Statistical Methods of Meta-Analysis
- Overview of the Process of Conducting Meta-analyses of the Diagnostic Test Accuracy
- An overview of systematic reviews of diagnostic tests accuracy
- Meta-Analysis of Diagnostic Test Accuracy
- Meta-analysis of diagnostic test accuracy studies with multiple thresholds for data integration