J Korean Med Sci.
2015 Oct;30(10):1405-1415. 10.3346/jkms.2015.30.10.1405.
Therapeutic Effects of Mesenchymal Stem Cells for Patients with Chronic Liver Diseases: Systematic Review and Meta-analysis
- Affiliations
-
- 1Research Institute for Nursing Science, Keimyung University, College of Nursing, Daegu, Korea.
- 2Cell Therapy and Tissue Engineering Center, Yonsei University, Wonju College of Medicine, Wonju, Korea. baiksk@yonsei.ac.kr
- 3Department of Internal Medicine, Yonsei University, Wonju College of Medicine, Wonju, Korea.
- 4Institute of Occupation and Environmental Medicine, Yonsei University, Wonju College of Medicine, Wonju, Korea.
- KMID: 2344172
- DOI: http://doi.org/10.3346/jkms.2015.30.10.1405
Abstract
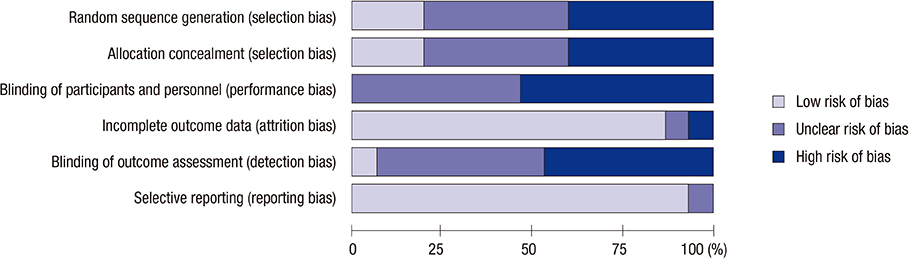
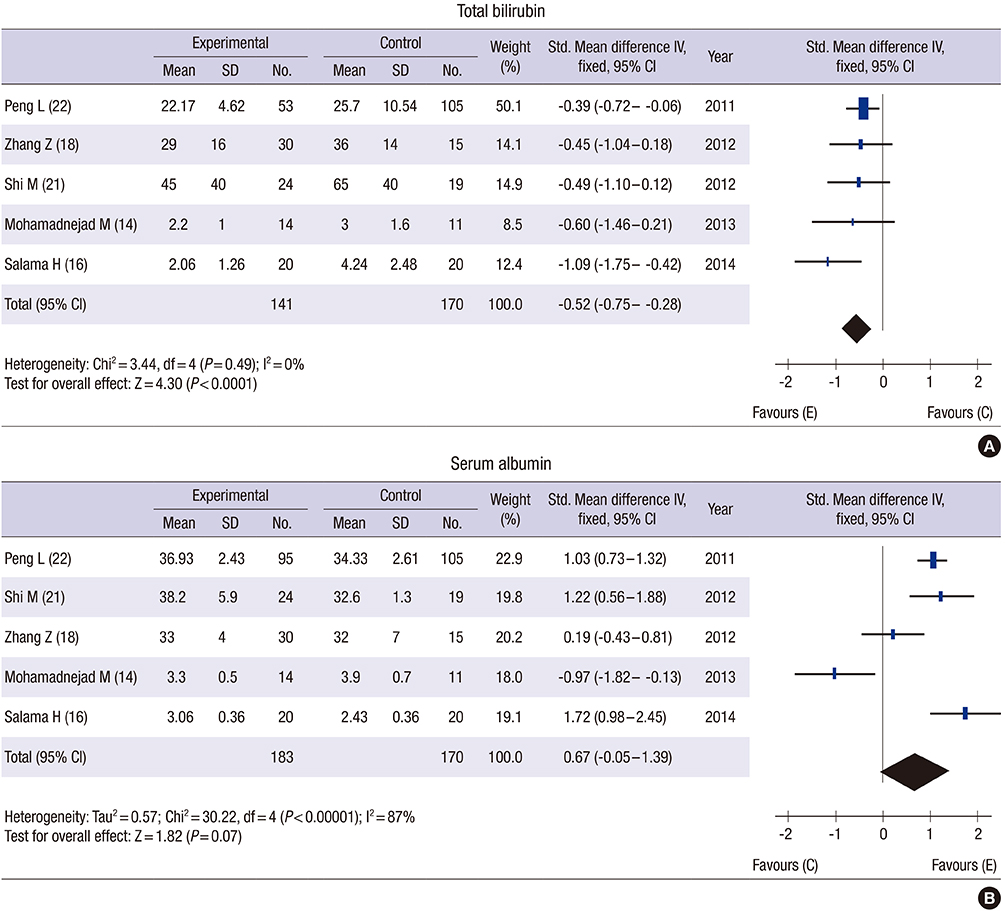
- Based on their ability to differentiate into multiple cell types including hepatocytes, the transplantation of mesenchymal stem cells (MSCs) has been suggested as an effective therapy for chronic liver diseases. The aim of this study was to evaluate the safety, efficacy and therapeutic effects of MSCs in patients with chronic liver disease through a literature-based examination. We performed a systematic review (SR) and meta-analysis (MA) of the literature using the Ovid-MEDLINE, EMBASE and Cochrane Library databases (up to November 2014) to identify clinical studies in which patients with liver diseases were treated with MSC therapy. Of the 568 studies identified by the initial literature search, we analyzed 14 studies and 448 patients based on our selection criteria. None of the studies reported the occurrence of statistically significant adverse events, side effects or complications. The majority of the analyzed studies showed improvements in liver function, ascites and encephalopathy. In particular, an MA showed that MSC therapy improved the total bilirubin level, the serum albumin level and the Model for End-stage Liver Disease (MELD) score after MSC treatment. Based on these results, MSC transplantation is considered to be safe for the treatment of chronic liver disease. However, although MSCs are potential therapeutic agents that may improve liver function, in order to obtain meaningful insights into their clinical efficacy, further robust clinical studies must be conducted to evaluate the clinical outcomes, such as histological improvement, increased survival and reduced liver-related complications, in patients with chronic liver disease.
MeSH Terms
Figure
Cited by 1 articles
-
Bone Marrow-Derived Mesenchymal Stem Cells Isolated from Patients with Cirrhosis and Healthy Volunteers Show Comparable Characteristics
Yoo Li Lim, Young Woo Eom, Su Jung Park, Taeui Hong, Seong Hee Kang, Soon Koo Baik, Kyu-Sang Park, Moon Young Kim
Int J Stem Cells. 2020;13(3):394-403. doi: 10.15283/ijsc20072.
Reference
-
1. Fallowfield JA, Iredale JP. Targeted treatments for cirrhosis. Expert Opin Ther Targets. 2004; 8:423–435.2. Chamuleau RA, Deurholt T, Hoekstra R. Which are the right cells to be used in a bioartificial liver? Metab Brain Dis. 2005; 20:327–335.3. Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001; 98:2396–2402.4. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000; 109:235–242.5. Young HE, Steele TA, Bray RA, Hudson J, Floyd JA, Hawkins K, Thomas K, Austin T, Edwards C, Cuzzourt J, et al. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001; 264:51–62.6. Kim G, Baik SK. Overview and recent trends of systematic reviews and meta-analyses in hepatology. Clin Mol Hepatol. 2014; 20:137–150.7. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. updated March 2011. accessed on 13 November 2014. Available at http://handbook.cochrane.org/.8. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–269. w649. Scottish Intercollegiate Guidelines Network. Methodology checklist 2: controlled trials. accessed on 15 November 2014. Available at http://www.sign.ac.uk/methodology/checklists.html.10. The Nordic Cochrane Centre. Review Manager (RevMan). Version 5.3 for Windows. Copenhagen: The Cochrane Collaboration;2013.11. Amin MA, Sabry D, Rashed LA, Aref WM, el-Ghobary MA, Farhan MS, Fouad HA, Youssef YA. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin Transplant. 2013; 27:607–612.12. Jang YO, Kim YJ, Baik SK, Kim MY, Eom YW, Cho MY, Park HJ, Park SY, Kim BR, Kim JW, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int. 2014; 34:33–41.13. Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, Telkabadi M, Atashi A, Honardoost M, Zali MR, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009; 21:1199–1205.14. Mohamadnejad M, Alimoghaddam K, Bagheri M, Ashrafi M, Abdollahzadeh L, Akhlaghpoor S, Bashtar M, Ghavamzadeh A, Malekzadeh R. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013; 33:1490–1496.15. Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, Baharvand H, Ghavamzadeh A, Malekzadeh R. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007; 10:459–466.16. Salama H, Zekri AR, Medhat E, Al Alim SA, Ahmed OS, Bahnassy AA, Lotfy MM, Ahmed R, Musa S. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther. 2014; 5:70.17. Wang L, Li J, Liu H, Li Y, Fu J, Sun Y, Xu R, Lin H, Wang S, Lv S, et al. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013; 28:85–92.18. Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J, Chen L, Lv S, Li Y, Yu S, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012; 27:112–120.19. Wang L, Han Q, Chen H, Wang K, Shan GL, Kong F, Yang YJ, Li YZ, Zhang X, Dong F, et al. Allogeneic bone marrow mesenchymal stem cell transplantation in patients with UDCA-resistant primary biliary cirrhosis. Stem Cells Dev. 2014; 23:2482–2489.20. El-Ansary M, Mogawer S, Abdel-Aziz I, Abdel-Hamid S. Phase I trial: mesenchymal stem cells transplantation in end stage liver disease. Stem Cell. 2010; 1:22–33.21. Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, Zhang A, Shi J, Chen L, Lv S, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012; 1:725–731.22. Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011; 54:820–828.23. El-Ansary M, Abdel-Aziz I, Mogawer S, Abdel-Hamid S, Hammam O, Teaema S, Wahdan M. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev. 2012; 8:972–981.24. Amer ME, El-Sayed SZ, El-Kheir WA, Gabr H, Gomaa AA, El-Noomani N, Hegazy M. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol. 2011; 23:936–941.25. Kim SJ, Park KC, Lee JU, Kim KJ, Kim DG. Therapeutic potential of adipose tissue-derived stem cells for liver failure according to the transplantation routes. J Korean Surg Soc. 2011; 81:176–186.26. Kim N, Cho SG. Clinical applications of mesenchymal stem cells. Korean J Intern Med. 2013; 28:387–402.27. Kim MD, Kim SS, Cha HY, Jang SH, Chang DY, Kim W, Suh-Kim H, Lee JH. Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis. Exp Mol Med. 2014; 46:e110.28. Jung J, Moon JW, Choi JH, Lee YW, Park SH, Kim GJ. Epigenetic alterations of IL-6/STAT3 signaling by placental stem cells promote hepatic regeneration in a rat model with CCl4-induced liver injury. Int J Stem Cells. 2015; 8:79–89.29. di Bonzo LV, Ferrero I, Cravanzola C, Mareschi K, Rustichell D, Novo E, Sanavio F, Cannito S, Zamara E, Bertero M, et al. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut. 2008; 57:223–231.30. Baertschiger RM, Serre-Beinier V, Morel P, Bosco D, Peyrou M, Clément S, Sgroi A, Kaelin A, Buhler LH, Gonelle-Gispert C. Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One. 2009; 4:e6657.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mesenchymal Stem Cells for the Treatment of Liver Disease: Present and Perspectives
- Adult Stem Cell Therapy in Chronic Liver Diseases
- Current Trends and Prospect of Cell Therapy using Hematopoietic Stem Cells
- Advanced Research on Stem Cell Therapy for Hepatic Diseases: Potential Implications of a Placenta-derived Mesenchymal Stem Cell-based Strategy
- Clinical application of stem cells in liver diseases