J Korean Med Sci.
2015 Sep;30(9):1232-1240. 10.3346/jkms.2015.30.9.1232.
Preserved Hippocampal Glucose Metabolism on 18F-FDG PET after Transplantation of Human Umbilical Cord Blood-derived Mesenchymal Stem Cells in Chronic Epileptic Rats
- Affiliations
-
- 1Department of Neurology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. jkkang@amc.seoul.kr
- 2Department of Neurology, Ulsan University Hospital, Ulsan, Korea.
- 3Adult Stem Cell Research, College of Veterinary Medicine, Seoul National University, Seoul, Korea.
- 4Department of Nuclear Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 5Department of Pathology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 6Department of Pediatrics, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 7The Asan Institute for Life Science, Seoul, Korea.
- KMID: 2344146
- DOI: http://doi.org/10.3346/jkms.2015.30.9.1232
Abstract
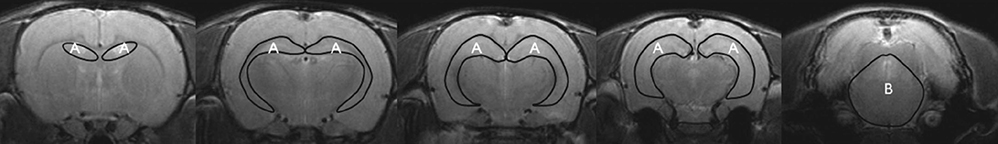
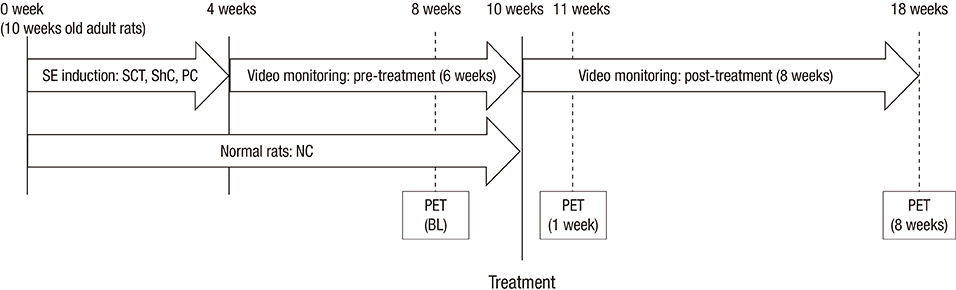
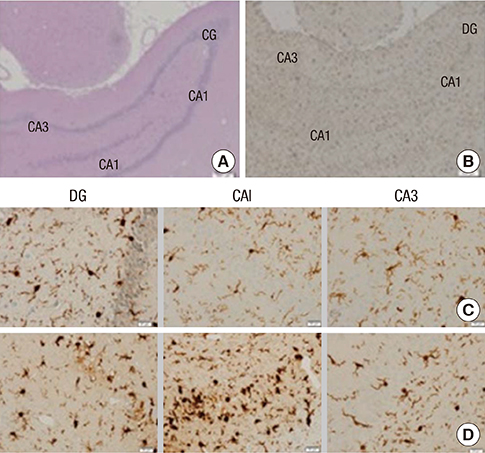
- Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) may be a promising modality for treating medial temporal lobe epilepsy. 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) is a noninvasive method for monitoring in vivo glucose metabolism. We evaluated the efficacy of hUCB-MSCs transplantation in chronic epileptic rats using FDG-PET. Rats with recurrent seizures were randomly assigned into three groups: the stem cell treatment (SCT) group received hUCB-MSCs transplantation into the right hippocampus, the sham control (ShC) group received same procedure with saline, and the positive control (PC) group consisted of treatment-negative epileptic rats. Normal rats received hUCB-MSCs transplantation acted as the negative control (NC). FDG-PET was performed at pre-treatment baseline and 1- and 8-week posttreatment. Hippocampal volume was evaluated and histological examination was done. In the SCT group, bilateral hippocampi at 8-week after transplantation showed significantly higher glucose metabolism (0.990 +/- 0.032) than the ShC (0.873 +/- 0.087; P < 0.001) and PC groups (0.858 +/- 0.093; P < 0.001). Histological examination resulted that the transplanted hUCB-MSCs survived in the ipsilateral hippocampus and migrated to the contralateral hippocampus but did not differentiate. In spite of successful engraftment, seizure frequency among the groups was not significantly different. Transplanted hUCB-MSCs can engraft and migrate, thereby partially restoring bilateral hippocampal glucose metabolism. The results suggest encouraging effect of hUCB-MSCs on restoring epileptic networks.
Keyword
MeSH Terms
-
Animals
Chronic Disease
Cord Blood Stem Cell Transplantation/*methods
Epilepsy, Temporal Lobe/*metabolism/pathology/*therapy
Fluorodeoxyglucose F18/*pharmacokinetics
Hippocampus/*metabolism/*pathology/surgery
Male
Mesenchymal Stem Cell Transplantation/methods
Radiopharmaceuticals/pharmacokinetics
Rats
Rats, Sprague-Dawley
Reproducibility of Results
Sensitivity and Specificity
Tissue Distribution
Treatment Outcome
Fluorodeoxyglucose F18
Radiopharmaceuticals
Figure
Reference
-
1. Hattiangady B, Rao MS, Shetty AK. Grafting of striatal precursor cells into hippocampus shortly after status epilepticus restrains chronic temporal lobe epilepsy. Exp Neurol. 2008; 212:468–481.2. Chu K, Kim M, Jung KH, Jeon D, Lee ST, Kim J, Jeong SW, Kim SU, Lee SK, Shin HS, et al. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine-induced status epilepticus in adult rats. Brain Res. 2004; 1023:213–221.3. Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS. Transplantation of human umbilical mesenchymal stem cells from Wharton's jelly after complete transection of the rat spinal cord. PLoS One. 2008; 3:e3336.4. van Eijsden P, Notenboom RG, Wu O, de Graan PN, van Nieuwenhuizen O, Nicolay K, Braun KP. In vivo 1H magnetic resonance spectroscopy, T2-weighted and diffusion-weighted MRI during lithium-pilocarpine-induced status epilepticus in the rat. Brain Res. 2004; 1030:11–18.5. Niessen HG, Angenstein F, Vielhaber S, Frisch C, Kudin A, Elger CE, Heinze HJ, Scheich H, Kunz WS. Volumetric magnetic resonance imaging of functionally relevant structural alterations in chronic epilepsy after pilocarpine-induced status epilepticus in rats. Epilepsia. 2005; 46:1021–1026.6. Kuo LW, Lee CY, Chen JH, Wedeen VJ, Chen CC, Liou HH, Tseng WY. Mossy fiber sprouting in pilocarpine-induced status epilepticus rat hippocampus: a correlative study of diffusion spectrum imaging and histology. Neuroimage. 2008; 41:789–800.7. Yakushev IY, Dupont E, Buchholz HG, Tillmanns J, Debus F, Cumming P, Heimann A, Fellgiebel A, Luhmann HJ, Landvogt C, et al. In vivo imaging of dopamine receptors in a model of temporal lobe epilepsy. Epilepsia. 2010; 51:415–422.8. Lee EM, Park GY, Im KC, Kim ST, Woo CW, Chung JH, Kim KS, Kim JS, Shon YM, Kim YI, et al. Changes in glucose metabolism and metabolites during the epileptogenic process in the lithium-pilocarpine model of epilepsy. Epilepsia. 2012; 53:860–869.9. Zhai Q, Gui J, Zhang Y, Qiao H. Children treated for epileptic encephalopathies show improved glucose metabolism. Pediatr Int. 2010; 52:883–887.10. Seo Y, Yang SR, Jee MK, Joo EK, Roh KH, Seo MS, Han TH, Lee SY, Ryu PD, Jung JW, et al. Human umbilical cord blood-derived mesenchymal stem cells protect against neuronal cell death and ameliorate motor deficits in Niemann Pick type C1 mice. Cell Transplant. 2011; 20:1033–1047.11. Wisenberg G, Lekx K, Zabel P, Kong H, Mann R, Zeman PR, Datta S, Culshaw CN, Merrifield P, Bureau Y, et al. Cell tracking and therapy evaluation of bone marrow monocytes and stromal cells using SPECT and CMR in a canine model of myocardial infarction. J Cardiovasc Magn Reson. 2009; 11:11.12. Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods. 1980; 3:129–149.13. Kornblum HI, Araujo DM, Annala AJ, Tatsukawa KJ, Phelps ME, Cherry SR. In vivo imaging of neuronal activation and plasticity in the rat brain by high resolution positron emission tomography (microPET). Nat Biotechnol. 2000; 18:655–660.14. Handforth A, Treiman DM. Functional mapping of the early stages of status epilepticus: a 14C-2-deoxyglucose study in the lithium-pilocarpine model in rat. Neuroscience. 1995; 64:1057–1073.15. Fernandes MJ, Dubé C, Boyet S, Marescaux C, Nehlig A. Correlation between hypermetabolism and neuronal damage during status epilepticus induced by lithium and pilocarpine in immature and adult rats. J Cereb Blood Flow Metab. 1999; 19:195–209.16. Dubé C, Boyet S, Marescaux C, Nehlig A. Relationship between neuronal loss and interictal glucose metabolism during the chronic phase of the lithium-pilocarpine model of epilepsy in the immature and adult rat. Exp Neurol. 2001; 167:227–241.17. Rschenschmidt C, Koch PG, Brstle O, Beck H. Functional properties of ES cell-derived neurons engrafted into the hippocampus of adult normal and chronically epileptic rats. Epilepsia. 2005; 46:174–183.18. Dedeurwaerdere S, Jupp B, O'Brien TJ. Positron Emission Tomography in basic epilepsy research: a view of the epileptic brain. Epilepsia. 2007; 48:56–64.19. Gao F, Guo Y, Zhang H, Wang S, Wang J, Wu JM, Chen Z, Ding MP. Anterior thalamic nucleus stimulation modulates regional cerebral metabolism: an FDG-MicroPET study in rats. Neurobiol Dis. 2009; 34:477–483.20. Guo Y, Gao F, Wang S, Ding Y, Zhang H, Wang J, Ding MP. In vivo mapping of temporospatial changes in glucose utilization in rat brain during epileptogenesis: an 18F-fluorodeoxyglucose-small animal positron emission tomography study. Neuroscience. 2009; 162:972–979.21. Duncan JS. Imaging and epilepsy. Brain. 1997; 120(Pt 2):339–377.22. Hotta SS. 18F-labeled 2-deoxy-2-fluoro-D-glucose positron-emission tomography scans for the localization of the epileptogenic foci. Health Technol Assess (Rockv). 1998; i–vi. 1–17.23. Wieser HG. PET and SPECT in epilepsy. Eur Neurol. 1994; 34:58–62.24. Ackermann RF, Engel J Jr, Phelps ME. Identification of seizure-mediating brain structures with the deoxyglucose method: studies of human epilepsy with positron emission tomography, and animal seizure models with contact autoradiography. Adv Neurol. 1986; 44:921–934.25. Benedek K, Juhász C, Chugani DC, Muzik O, Chugani HT. Longitudinal changes in cortical glucose hypometabolism in children with intractable epilepsy. J Child Neurol. 2006; 21:26–31.26. Lee JS, Asano E, Muzik O, Chugani DC, Juhász C, Pfund Z, Philip S, Behen M, Chugani HT. Sturge-Weber syndrome: correlation between clinical course and FDG PET findings. Neurology. 2001; 57:189–195.27. Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002; 20:1103–1110.28. Pluchino S, Furlan R, Martino G. Cell-based remyelinating therapies in multiple sclerosis: evidence from experimental studies. Curr Opin Neurol. 2004; 17:247–255.29. Love Z, Wang F, Dennis J, Awadallah A, Salem N, Lin Y, Weisenberger A, Majewski S, Gerson S, Lee Z. Imaging of mesenchymal stem cell transplant by bioluminescence and PET. J Nucl Med. 2007; 48:2011–2020.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Percutaneous transplantation of human umbilical cord-derived mesenchymal stem cells in a dog suspected to have fibrocartilaginous embolic myelopathy
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood
- Difference in HLA-DR Expression of Human Umbilical Cord Blood Derived Mesenchymal Stem Cells after Tri-lineage Differentiation
- Stem Cell Transplantation in Umbilical Cord Blood(I) Expansion Effects of Stem Cells in Umbilical Cord Blood with Various Hematopoietic Growth Factors
- Umbilical Cord Blood Transplantation