Korean J Urol.
2015 Oct;56(10):689-694. 10.4111/kju.2015.56.10.689.
The effect of continuous androgen deprivation treatment on prostate cancer patients as compared with intermittent androgen deprivation treatment
- Affiliations
-
- 1Department of Urology, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea. hongkooha@pusan.ac.kr
- 2Biomedical Research Institute, Pusan National University Hospital, Busan, Korea.
- KMID: 2344111
- DOI: http://doi.org/10.4111/kju.2015.56.10.689
Abstract
- PURPOSE
To investigate the efficacy of androgen deprivation treatment (ADT) between continuous and intermittent ADT.
MATERIALS AND METHODS
Between January 2006 and May 2015, 603 patients were selected and divided into continuous ADT (CADT) (n=175) and intermittent ADT (IADT) (n=428) groups. The median follow-up in this study was 48.19 (1.0-114.0) months. The primary end point was time to castration resistant prostate cancer (CRPC). The types of ADT were monotherapy and maximal androgen blockade (i.e., luteinizing hormone-releasing hormone agonist and antiandrogen).
RESULTS
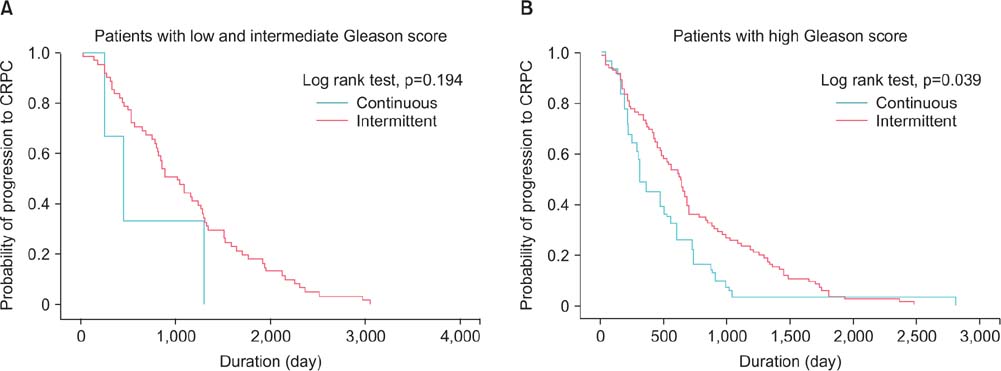
The characteristics of patients showed no significant differences between the CADT and IADT groups, except for the Gleason score (p<0.001). The median time to CRPC of all enrolled patients with ADT was 20.60±1.60 months. The median time to CRPC was 11.20±1.31 months in the CADT group as compared with 22.60±2.08 months in the IADT group. In multivariate analysis, percentage of positive core (p=0.047; hazard ratio [HR], 0.976; 95% confidence interval [CI], 0.953-1.000), Gleason score (p=0.007; HR, 1.977; 95% CI, 1.206-3.240), lymph node metastasis (p=0.030; HR, 0.498; 95% CI, 0.265-0.936), bone metastasis (p=0.028; HR, 1.921; 95% CI, 1.072-3.445), and CADT vs. IADT (p=0.003; HR, 0.254; 95% CI. 0.102-0.633) were correlated with the duration of progression to CRPC. The IADT group presented a significantly longer median time to CRPC compared with the CADT group. Additionally, patients in the IADT group showed a longer duration in median time to CRPC in subgroup analysis according to the Gleason score.
CONCLUSIONS
This study found that IADT produces a longer duration in median time to CRPC than does CADT.
Keyword
MeSH Terms
-
Adenocarcinoma/*drug therapy/pathology/secondary
Aged
Aged, 80 and over
Androgen Antagonists/*administration & dosage/therapeutic use
Antineoplastic Agents, Hormonal/*administration & dosage/therapeutic use
Disease Progression
Drug Administration Schedule
Follow-Up Studies
Humans
Lymphatic Metastasis
Male
Middle Aged
Neoplasm Grading
Prostatic Neoplasms/*drug therapy/pathology
Prostatic Neoplasms, Castration-Resistant/drug therapy/pathology
Retrospective Studies
Treatment Outcome
Androgen Antagonists
Antineoplastic Agents, Hormonal
Figure
Reference
-
1. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014; 65:124–137.2. Schroder F, Crawford ED, Axcrona K, Payne H, Keane TE. Androgen deprivation therapy: past, present and future. BJU Int. 2012; 109:Suppl 6. 1–12.3. Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, et al. EAU guidelines on prostate cancer. Eur Urol. 2008; 53:68–80.4. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247.5. Lepor H, Shore ND. LHRH Agonists for the treatment of prostate cancer: 2012. Rev Urol. 2012; 14:1–12.6. Denis L, Murphy GP. Overview of phase III trials on combined androgen treatment in patients with metastatic prostate cancer. Cancer. 1993; 72:12 Suppl. 3888–3895.7. Conti PD, Atallah AN, Arruda H, Soares BG, El Dib RP, Wilt TJ. Intermittent versus continuous androgen suppression for prostatic cancer. Cochrane Database Syst Rev. 2007; (4):CD005009.8. Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2013; 189:1 Suppl. S34–S42.9. Sharifi N, Gulley JL, Dahut WL. An update on androgen deprivation therapy for prostate cancer. Endocr Relat Cancer. 2010; 17:R305–R315.10. Calais da Silva FE, Bono AV, Whelan P, Brausi M, Marques Queimadelos A, Martin JA, et al. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the South European Uroncological Group. Eur Urol. 2009; 55:1269–1277.11. Tunn UW, Canepa G, Kochanowsky A, Kienle E. Testosterone recovery in the off-treatment time in prostate cancer patients undergoing intermittent androgen deprivation therapy. Prostate Cancer Prostatic Dis. 2012; 15:296–302.12. Crook JM, O'Callaghan CJ, Duncan G, Dearnaley DP, Higano CS, Horwitz EM, et al. Intermittent androgen suppression forrising PSA level after radiotherapy. N Engl J Med. 2012; 367:895–903.13. Salonen AJ, Taari K, Ala-Opas M, Viitanen J, Lundstedt S, Tammela TL, et al. Advanced prostate cancer treated with intermittent or continuous androgen deprivation in the randomised FinnProstate Study VII: quality of life and adverse effects. Eur Urol. 2013; 63:111–120.14. Salonen AJ, Taari K, Ala-Opas M, Viitanen J, Lundstedt S, Tammela TL, et al. The FinnProstate Study VII: intermittent versus continuous androgen deprivation in patients with advanced prostate cancer. J Urol. 2012; 187:2074–2081.15. Langenhuijsen JF, Badhauser D, Schaaf B, Kiemeney LA, Witjes JA, Mulders PF. Continuous vs. intermittent androgen deprivation therapy for metastatic prostate cancer. Urol Oncol. 2013; 31:549–556.16. Irani J, Celhay O, Hubert J, Bladou F, Ragni E, Trape G, et al. Continuous versus six months a year maximal androgen blockade in the management of prostate cancer: a randomised study. Eur Urol. 2008; 54:382–391.17. Calais da Silva F, Calais da Silva FM, Gonçalves F, Santos A, Kliment J, Whelan P, et al. Locally advanced and metastatic prostate cancer treated with intermittent androgen monotherapy or maximal androgen blockade: results from a randomised phase 3 study by the South European Uroncological Group. Eur Urol. 2014; 66:232–239.18. Mottet N, Van Damme J, Loulidi S, Russel C, Leitenberger A, Wolff JM, et al. Intermittent hormonal therapy in the treatment of metastatic prostate cancer: a randomized trial. BJU Int. 2012; 110:1262–1269.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intermittent Androgen Deprivation with Goserelin and Flutamide for Prostate Cancer: a Pilot Study
- Differentiation Related Gene (Drg-1) as a Molecular Marker during the Treatment of in vitro Intermittent Androgen Deprivation in prostate Cancer
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- Current Concepts in Androgen Deprivation Therapy
- Intermittent Androgen Deprivation(IAD) with Cyproterone Acetate Monotherapy for Prostate Cancer: A preliminary report