J Korean Ophthalmol Soc.
2014 Jul;55(7):978-983.
Effect of Cyclosporin A on Tear Film and Corneal Aberration after Cataract Surgery
- Affiliations
-
- 1The Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea. TIKIM@yuhs.ac
Abstract
- PURPOSE
To evaluate the efficacy of 0.05% cyclosporine A on tear film parameters and corneal aberration after cataract surgery.
METHODS
Patients who underwent cataract surgery were divided into 2 groups. Patients in Group I (23 eyes) were treated with cyclosporine A from 1 week before surgery to 3 months after surgery. Patients in Group II (24 eyes) underwent surgery without cyclosporine treatment. Tear film break-up time (BUT), Schirmer's test I, Oxford scheme, Ocular surface disease index (OSDI), and corneal aberrations were evaluated before surgery and at 1 and 3 months after surgery.
RESULTS
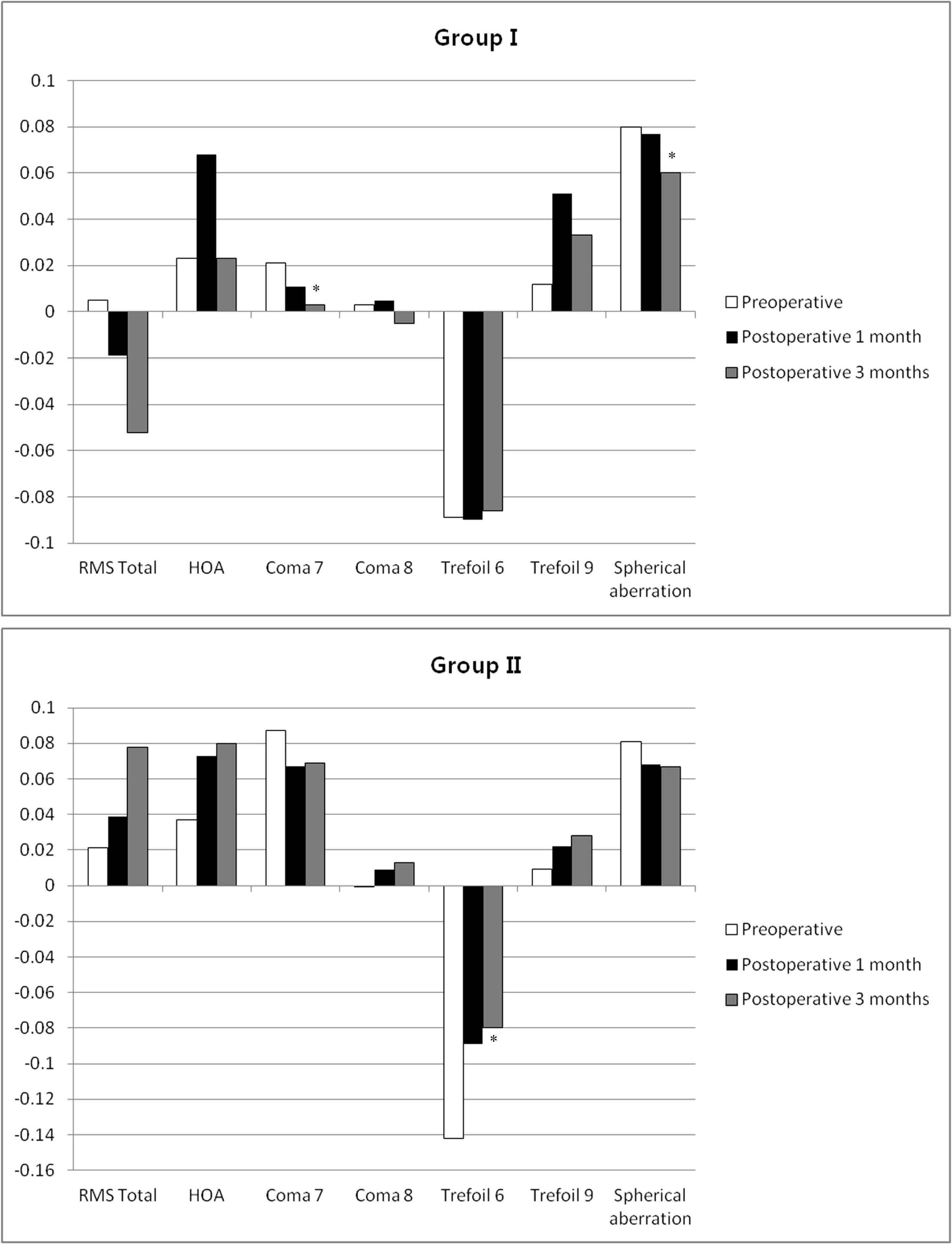
In Group I, BUT was significantly improved at 3 months (p = 0.026) after surgery compared with the preoperative value. OSDI decreased significantly at 1 (p = 0.033) and 3 months (p = 0.003) after surgery compared with the preoperative value. However, there were no significant differences between preoperative and postoperative values of BUT and OSDI in Group II. Schirmer's test results and the Oxford scheme were not significantly changed in either group. Preoperative root mean square (RMS) total values were not different between the 2 groups, but was different at postoperative 3 months (p = 0.015). Group I had a significantly lower value for total RMS than Group II. In Group I, Coma 7 (Z3(-1)) (p = 0.018) and spherical aberration (Z4(0)) (p = 0.031) were significantly decreased after surgery. In Group II, Trefoil 6 (Z3(-3)) (p = 0.033) was significantly increased after surgery.
CONCLUSIONS
0.05% cyclosporine A may be effective for improving dry eye syndrome and corneal aberration after cataract surgery.
Keyword
Figure
Reference
-
References
1. Apostol S, Filip M, Dragne C, Filip A. Dry eye syndrome. Etiological and therapeutic aspects. Oftalmología. 2003; 59:28–31.2. Sheppard JD. Guidelines for the treatment of chronic dry eye disease. Manag Care. 2003; 12:20–5.3. Li XM, Hu L, Hu J, Wang W. Investigation of dry eye disease and analysis of the pathogenic factors in patients after cataract surgery. Cornea. 2007; 26:S16–20.
Article4. Albietz JM, Lenton LM. Management of the ocular surface and tear film before, during, and after laser in situ keratomileusis. J Refract Surg. 2004; 20:62–71.
Article5. Begley CG, Caffery B, Nichols K, et al. Results of a dry eye questionnaire from optometric practices in North America. Adv Exp Med Biol. 2002; 506:1009–16.
Article6. Khanal S, Tomlinson A, Esakowitz L, et al. Changes in corneal sensitivity and tear physiology after phacoemulsification. Ophthalmic Physiol Opt. 2008; 28:127–34.
Article7. Oh T, Jung Y, Chang D, et al. Changes in the tear film and ocular surface after cataract surgery. Jpn J Ophthalmol. 2012; 56:113–8.
Article8. Albarran C, Pons AM, Lorente A, et al. Influence of the tear film on optical quality ofthe eye. Cont Lens Anterior Eye. 1997; 20:129–35.9. Denoyer A, Rabut G, Baudouin C. Tear film aberration dynamics and vision-related quality of life in patients with dry eye disease. Ophthalmology. 2012; 119:1811–8.
Article10. Choi SH, Shin YI. Changes in higher order aberration according to tear-film instability analyzed by continuous measurement using wavefront. J Korean Ophthalmol Soc. 2012; 53:1076–80.
Article11. Pflugfelder SC, Tseng SC, Sanabria O, et al. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998; 17:38–56.
Article12. Byun YS, Jeon EJ, Chung SK. Clinical effect of cyclosporine 0.05% eye drops in dry eye syndrome patients. J Korean Ophthalmol Soc. 2008; 49:1583–8.
Article13. Hom MM. Use of cyclosporine 0.05% ophthalmic emulsion for contact lens-intolerant patients. Eye Contact Lens. 2006; 32:109–11.
Article14. Byun YS, Rho CR, Cho K, et al. Cyclosporine 0.05% ophthalmic emulsion for dry eye in Korea: a prospective, multicenter, open-label, surveillance study. Korean J Ophthalmol. 2011; 25:369–74.
Article15. Donnenfeld ED, Solomon R, Roberts CW, et al. Cyclosporine 0.05% to improve visual outcomes after multifocal intraocular lens implantation. J Cataract Refract Surg. 2010; 36:1095–100.
Article16. Chung YW, Oh TH, Chung SK. The effect of topical cyclosporine 0.05% on dry eye after cataract surgery. Korean J Ophthalmol. 2013; 27:167–71.
Article17. O'Brien PD, Collum LM. Dry eye: diagnosis and current treatment strategies. Curr Allergy Asthma Rep. 2004; 4:314–9.18. Stern ME, Pflugfelder SC. Inflammation in dry eye. Ocul Surf. 2004; 2:124–30.
Article19. Moon SJ, Lee DJ, Lee KH. Induced astigmatism and high-order aberrations after 1.8-mm, 2.2-mm and 3.0-mm coaxial phacoemulsi- fication incisions. J Korean Ophthalmol Soc. 2011; 52:407–13.20. Oh HC, Lee DJ, Park WC. Changes of the corneal aberration following cataract surgery. J Korean Ophthalmol Soc. 2009; 50:518–22.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Wavefront Aberration Changes after the Instillation of Artificial Tear in Dry Eyes
- The Change of Corneal Sensation and Tear Film Stability after Cataract Surgery in Diabetic Patients
- Changes of the Corneal Aberration Following Cataract Surgery
- Change of Lipid Layer in Tear Film after Cataract Surgery
- Comparison between Anterior Corneal Aberration and Ocular Aberration in Laser Refractive Surgery