J Korean Soc Hypertens.
2012 Sep;18(3):75-87.
Blood Pressure Variability and Vascular Dysfunction in Essential Hypertension
- Affiliations
-
- 1Department of Cardiology, The Toki Municipal General Hospital, Toki, Japan. kenji@med.nagoya-u.ac.jp
- 2Department of Cardiology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
- 3Department of Cardiology, Yanbian University Hospital, Yanji, China.
Abstract
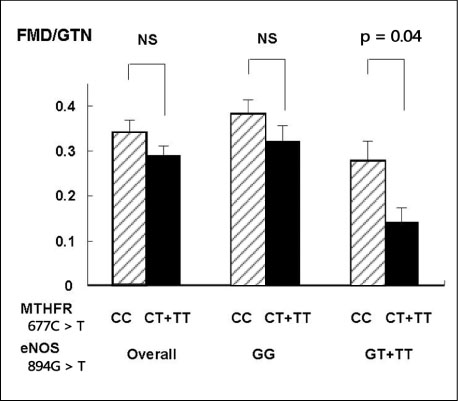
- There are several aspects of blood pressure. Clinically, how to best assess blood pressure average and variability is still a matter of the ongoing debate. Besides office blood pressure, we must pay more careful attention focused on hypertension with blood pressure fluctuation. Impaired endothelial function is intimately associated with the development of hypertension and atherosclerosis. In this review, we describe the relation between endothelial dysfunction and hypertension, the effect of gene polymorphism on endothelial dysfunction, the effects of antihypertensive agents and dietary supplementation on impaired endothelial function in hypertension. In order to predict the future atherosclerosis and cardiovascular events in subjects with hypertension, the adequate assessment of endothelial function is one of the most reliable markers. Furthermore, we discuss the close relationship between blood pressure variability and endothelial function. Blood pressure variability during a day or a week is an important, new risk factor for cardiovascular disease and restoring impaired endothelial function might be a target to prevent blood pressure variation and future cardiovascular events.
Keyword
MeSH Terms
Figure
Reference
-
1. Wolf-Maier K, Cooper RS, Kramer H, Banegas JR, Giampaoli S, Joffres MR, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004. 43:10–17.
Article2. Kaplan NM, Opie LH. Controversies in hypertension. Lancet. 2006. 367:168–176.
Article3. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003. 289:2560–2572.
Article4. National Institute for Health and Clinical Excellence. Hypertension: clinical management of primary hypertension in adults (update)--clinical guideline 127 [Internet]. 2011. cited 2012 Jun 30. London: NICE;Available from: http://guidance.nice.org.uk/CG127.5. Krause T, Lovibond K, Caulfield M, McCormack T, Williams B. Guideline Development Group. Management of hypertension: summary of NICE guidance. BMJ. 2011. 343:d4891.
Article6. Mancia G, Zanchetti A, Agabiti-Rosei E, Benemio G, De Cesaris R, Fogari R, et al. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment-induced regression of left ventricular hypertrophy. SAMPLE Study Group. Study on Ambulatory Monitoring of Blood Pressure and Lisinopril Evaluation. Circulation. 1997. 95:1464–1470.7. Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Bjorklund-Bodegard K, et al. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010. 55:1049–1057.
Article8. Matsui Y, Ishikawa J, Eguchi K, Shibasaki S, Shimada K, Kario K. Maximum value of home blood pressure: a novel indicator of target organ damage in hypertension. Hypertension. 2011. 57:1087–1093.9. Pocock SJ, McCormack V, Gueyffier F, Boutitie F, Fagard RH, Boissel JP. A score for predicting risk of death from cardiovascular disease in adults with raised blood pressure, based on individual patient data from randomized controlled trials. BMJ. 2001. 323:75–81.10. Winkelmayer WC, Stampfer MJ, Willett WC, Curhan GC. Habitual caffeine intake and the risk of hypertension in women. JAMA. 2005. 294:2330–2335.
Article11. Guyton AC. Circulatory physiology III: arterial pressure and hypertension. 1980. Philadelphia, PA: Saunders.12. Guyton AC. Blood pressure control: special role of the kidneys and body fluids. Science. 1991. 252:1813–1816.13. Barker DJ, Martyn CN. The fetal origins of hypertension. Adv Nephrol Necker Hosp. 1997. 26:65–72.14. Manalich R, Reyes L, Herrera M, Melendi C, Fundora I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 2000. 58:770–773.15. Cheng XW, Kuzuya M, Sasaki T, Inoue A, Hu L, Song H, et al. Inhibition of mineralocorticoid receptor is a renoprotective effect of the 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor pitavastatin. J Hypertens. 2011. 29:542–552.
Article16. Williams RR, Hunt SC, Hasstedt SJ, Hopkins PN, Wu LL, Berry TD, et al. Are there interactions and relations between genetic and environmental factors predisposing to high blood pressure? Hypertension. 1991. 18:3 Suppl. I29–I37.
Article17. Victor RG, Kaplan NM. Libby P, Braunwald E, Bonow RO, Mann DL, Zipes DP, editors. Systemic hypertension: mechanisms and diagnosis. Braunwald's heart disease: a textbook of cardiovascular medicine. 2008. 8th ed. Philadelphia, PA: Saunders;1195–1205.18. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980. 288:373–376.
Article19. Vita JA, Keaney JF Jr, Larson MG, Keyes MJ, Massaro JM, Lipinska I, et al. Brachial artery vasodilator function and systemic inflammation in the Framingham Offspring Study. Circulation. 2004. 110:3604–3609.
Article20. Reddy KG, Nair RN, Sheehan HM, Hodgson JM. Evidence that selective endothelial dysfunction may occur in the absence of angiographic or ultrasound atherosclerosis in patients with risk factors for atherosclerosis. J Am Coll Cardiol. 1994. 23:833–843.
Article21. Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000. 101:948–954.
Article22. Healy B. Endothelial cell dysfunction: an emerging endocrinopathy linked to coronary disease. J Am Coll Cardiol. 1990. 16:357–358.
Article23. Ross R. The pathogenesis of atherosclerosis: an update. N Engl J Med. 1986. 314:488–500.24. Weil BR, Stauffer BL, Greiner JJ, DeSouza CA. Prehypertension is associated with impaired nitric oxide-mediated endothelium-dependent vasodilation in sedentary adults. Am J Hypertens. 2011. 24:976–981.
Article25. Kissel CK, Anderson TJ. Role of endothelin-1 and endothelial dysfunction in prehypertension. Can J Cardiol. 2012. 28:251–253.
Article26. Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999. 340:115–126.27. Naruse K, Shimizu K, Muramatsu M, Toki Y, Miyazaki Y, Okumura K, et al. Long-term inhibition of NO synthesis promotes atherosclerosis in the hypercholesterolemic rabbit thoracic aorta. PGH2 does not contribute to impaired endothelium-dependent relaxation. Arterioscler Thromb. 1994. 14:746–752.
Article28. Wang J, Felux D, VandeBerg J, Wang XL. Discordance of endothelial nitric oxide synthase in the arterial wall and its circulating products in baboons: interactions with redox metabolism. Eur J Clin Invest. 2003. 33:288–295.
Article29. Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet. 2011. 43:531–538.
Article30. International Consortium for Blood Pressure Genome-Wide Association Studies. Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011. 478:103–109.
Article31. Wain LV, Verwoert GC, O'Reilly PF, Shi G, Johnson T, Johnson AD, et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011. 43:1005–1011.32. Johnson T, Gaunt TR, Newhouse SJ, Padmanabhan S, Tomaszewski M, Kumari M, et al. Blood pressure loci identified with a gene-centric array. Am J Hum Genet. 2011. 89:688–700.33. Salvi E, Kutalik Z, Glorioso N, Benaglio P, Frau F, Kuznetsova T, et al. Genomewide association study using a high-density single nucleotide polymorphism array and case-control design identifies a novel essential hypertension susceptibility locus in the promoter region of endothelial NO synthase. Hypertension. 2012. 59:248–255.
Article34. Luizon MR, Metzger IF, Lacchini R, Tanus-Santos JE. Endothelial nitric oxide synthase polymorphism rs3918226 associated with hypertension does not affect plasma nitrite levels in healthy subjects. Hypertension. 2012. 59:e52.
Article35. Green D. Point: flow-mediated dilation does reflect nitric oxide-mediated endothelial function. J Appl Physiol. 2005. 99:1233–1234.
Article36. Lazdam M, Lewandowski AJ, Kylintireas I, Cunnington C, Diesch J, Francis J, et al. Impaired endothelial responses in apparently healthy young people associated with subclinical variation in blood pressure and cardiovascular phenotype. Am J Hypertens. 2012. 25:46–53.
Article37. Chung WY, Kim KI, Chang HJ, Cho YS, Youn TJ, Chae IH, et al. The effects of amlodipine besylate and hydrochlorothiazide on brachial arterial vosomotor function in hypertensive patients. J Korean Soc Hypertens. 2005. 11:10–17.38. Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002. 106:653–658.
Article39. Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001. 104:191–196.
Article40. Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002. 105:1567–1572.41. Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003. 41:1769–1775.42. Imamura A, Okumura K, Matsui H, Mizuno T, Ogawa Y, Imai H, et al. Endothelial nitric oxide synthase and methylenetetrahydrofolate reductase gene polymorphisms are associated with endothelial dysfunction in young, healthy men. Can J Cardiol. 2004. 20:1229–1234.43. Imamura A, Takahashi R, Murakami R, Kataoka H, Cheng XW, Numaguchi Y, et al. The effects of endothelial nitric oxide synthase gene polymorphisms on endothelial function and metabolic risk factors in healthy subjects: the significance of plasma adiponectin levels. Eur J Endocrinol. 2008. 158:189–195.
Article44. Asakimori Y, Yorioka N, Taniguchi Y, Ito T, Ogata S, Kyuden Y, et al. T(-786)→C polymorphism of the endothelial nitric oxide synthase gene influences the progression of renal disease. Nephron. 2002. 91:747–751.45. Hassan A, Gormley K, O'Sullivan M, Knight J, Sham P, Vallance P, et al. Endothelial nitric oxide gene haplotypes and risk of cerebral small-vessel disease. Stroke. 2004. 35:654–659.
Article46. Kathiresan S, Larson MG, Vasan RS, Guo CY, Vita JA, Mitchell GF, et al. Common genetic variation at the endothelial nitric oxide synthase locus and relations to brachial artery vasodilator function in the community. Circulation. 2005. 112:1419–1427.
Article47. Vasan RS, Larson MG, Aragam J, Wang TJ, Mitchell GF, Kathiresan S, et al. Genome-wide association of echocardiographic dimensions, brachial artery endothelial function and treadmill exercise responses in the Framingham Heart Study. BMC Med Genet. 2007. 8:Suppl 1. S2.
Article48. Tesauro M, Thompson WC, Rogliani P, Qi L, Chaudhary PP, Moss J. Intracellular processing of endothelial nitric oxide synthase isoforms associated with differences in severity of cardiopulmonary diseases: cleavage of proteins with aspartate vs. glutamate at position 298. Proc Natl Acad Sci U S A. 2000. 97:2832–2835.
Article49. Fairchild TA, Fulton D, Fontana JT, Gratton JP, McCabe TJ, Sessa WC. Acidic hydrolysis as a mechanism for the cleavage of the Glu(298)→Asp variant of human endothelial nitric-oxide synthase. J Biol Chem. 2001. 276:26674–26679.50. Nakayama M, Yasue H, Yoshimura M, Shimasaki Y, Kugiyama K, Ogawa H, et al. T-786-->C mutation in the 5'-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm. Circulation. 1999. 99:2864–2870.51. Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009. 41:666–676.52. Nishio K, Goto Y, Kondo T, Ito S, Ishida Y, Kawai S, et al. Serum folate and methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism adjusted for folate intake. J Epidemiol. 2008. 18:125–131.
Article53. Imamura A, Murakami R, Takahashi R, Cheng XW, Numaguchi Y, Murohara T, et al. Low folate levels may be an atherogenic factor regardless of homocysteine levels in young healthy nonsmokers. Metabolism. 2010. 59:728–733.
Article54. Zhang X, Li H, Jin H, Ebin Z, Brodsky S, Goligorsky MS. Effects of homocysteine on endothelial nitric oxide production. Am J Physiol Renal Physiol. 2000. 279:F671–F678.
Article55. Verhaar MC, Stroes E, Rabelink TJ. Folates and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002. 22:6–13.
Article56. Voutilainen S, Morrow JD, Roberts LJ 2nd, Alfthan G, Alho H, Nyyssonen K, et al. Enhanced in vivo lipid peroxidation at elevated plasma total homocysteine levels. Arterioscler Thromb Vasc Biol. 1999. 19:1263–1266.
Article57. Rees DD, Palmer RM, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989. 86:3375–3378.
Article58. Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glomerular damage. J Clin Invest. 1992. 90:278–281.
Article59. Ribeiro MO, Antunes E, de Nucci G, Lovisolo SM, Zatz R. Chronic inhibition of nitric oxide synthesis: a new model of arterial hypertension. Hypertension. 1992. 20:298–303.
Article60. Salazar FJ, Pinilla JM, Lopez F, Romero JC, Quesada T. Renal effects of prolonged synthesis inhibition of endothelium-derived nitric oxide. Hypertension. 1992. 20:113–117.
Article61. Tomida T, Numaguchi Y, Nishimoto Y, Tsuzuki M, Hayashi Y, Imai H, et al. Inhibition of COX-2 prevents hypertension and proteinuria associated with a decrease of 8-iso-PGF2alpha formation in L-NAME-treated rats. J Hypertens. 2003. 21:601–609.62. Creager MA, Gallagher SJ, Girerd XJ, Coleman SM, Dzau VJ, Cooke JP. L-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest. 1992. 90:1248–1253.
Article63. Clarkson P, Adams MR, Powe AJ, Donald AE, McCredie R, Robinson J, et al. Oral L-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. J Clin Invest. 1996. 97:1989–1994.
Article64. Pieper GM, Siebeneich W, Dondlinger LA. Short-term oral administration of L-arginine reverses defective endothelium-dependent relaxation and cGMP generation in diabetes. Eur J Pharmacol. 1996. 317:317–320.
Article65. Dong JY, Qin LQ, Zhang Z, Zhao Y, Wang J, Arigoni F, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011. 162:959–965.
Article66. Bode-Boger SM, Scalera F, Ignarro LJ. The L-arginine paradox: importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol Ther. 2007. 114:295–306.67. Boger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the "L-arginine paradox" and acts as a novel cardiovascular risk factor. J Nutr. 2004. 134:10 Suppl. 2842S–2847S.68. Alvares TS, Conte-Junior CA, Silva JT, Flosi Paschoalin VM. Acute L-Arginine supplementation does not increase nitric oxide production in healthy subjects. Nutr Metab (Lond). 2012. 9:54.
Article69. Lundberg JO, Weitzberg E. NO generation from nitrite and its role in vascular control. Arterioscler Thromb Vasc Biol. 2005. 25:915–922.
Article70. Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, et al. The emerging biology of the nitrite anion. Nat Chem Biol. 2005. 1:308–314.
Article71. Carlstrom M, Persson AE, Larsson E, Hezel M, Scheffer PG, Teerlink T, et al. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc Res. 2011. 89:574–585.72. Hobbs AJ. Soluble guanylate cyclase: the forgotten sibling. Trends Pharmacol Sci. 1997. 18:484–491.
Article73. Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, et al. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010. 56:274–281.74. Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006. 355:2792–2793.
Article75. Shahin Y, Khan JA, Samuel N, Chetter I. Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: a meta-analysis of randomized controlled trials. Atherosclerosis. 2011. 216:7–16.76. Mourad JJ, Waeber B, Zannad F, Laville M, Duru G, Andrejak M, et al. Comparison of different therapeutic strategies in hypertension: a low-dose combination of perindopril/indapamide versus a sequential monotherapy or a stepped-care approach. J Hypertens. 2004. 22:2379–2386.77. Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999. 399:597–601.
Article78. Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, et al. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000. 101:1539–1545.79. Steinberg HO, Baron AD. Vascular function, insulin resistance and fatty acids. Diabetologia. 2002. 45:623–634.
Article80. Sarafidis PA, Bakris GL. Review: insulin and endothelin: an interplay contributing to hypertension development? J Clin Endocrinol Metab. 2007. 92:379–385.81. Iglarz M, Touyz RM, Amiri F, Lavoie MF, Diep QN, Schiffrin EL. Effect of peroxisome proliferator-activated receptor-alpha and -gamma activators on vascular remodeling in endothelin-dependent hypertension. Arterioscler Thromb Vasc Biol. 2003. 23:45–51.82. Takeda K, Ichiki T, Tokunou T, Iino N, Takeshita A. 15-Deoxy-delta 12,14-prostaglandin J2 and thiazolidinediones activate the MEK/ERK pathway through phosphatidylinositol 3-kinase in vascular smooth muscle cells. J Biol Chem. 2001. 276:48950–48955.83. Pistrosch F, Passauer J, Fischer S, Fuecker K, Hanefeld M, Gross P. In type 2 diabetes, rosiglitazone therapy for insulin resistance ameliorates endothelial dysfunction independent of glucose control. Diabetes Care. 2004. 27:484–490.
Article84. Murakami T, Mizuno S, Ohsato K, Moriuchi I, Arai Y, Nio Y, et al. Effects of troglitazone on frequency of coronary vasospastic-induced angina pectoris in patients with diabetes mellitus. Am J Cardiol. 1999. 84:92–94. A8.
Article85. Chan SH, Wu KL, Kung PS, Chan JY. Oral intake of rosiglitazone promotes a central antihypertensive effect via upregulation of peroxisome proliferator-activated receptor-gamma and alleviation of oxidative stress in rostral ventrolateral medulla of spontaneously hypertensive rats. Hypertension. 2010. 55:1444–1453.86. Dorafshar AH, Moodley K, Khoe M, Lyon C, Bryer-Ash M. Pioglitazone improves superoxide dismutase mediated vascular reactivity in the obese Zucker rat. Diab Vasc Dis Res. 2010. 7:20–27.
Article87. Raphael KL, Strait KA, Stricklett PK, Baird BC, Piontek K, Germino GG, et al. Effect of pioglitazone on survival and renal function in a mouse model of polycystic kidney disease. Am J Nephrol. 2009. 30:468–473.
Article88. Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, et al. Prevalence of hypertension in the US adult population: results from the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995. 25:305–313.89. Opie LH, Seedat YK. Hypertension in sub-Saharan African populations. Circulation. 2005. 112:3562–3568.
Article90. Smith WR, Betancourt JR, Wynia MK, Bussey-Jones J, Stone VE, Phillips CO, et al. Recommendations for teaching about racial and ethnic disparities in health and health care. Ann Intern Med. 2007. 147:654–665.
Article91. Taherzadeh Z, Brewster LM, van Montfrans GA, VanBavel E. Function and structure of resistance vessels in black and white people. J Clin Hypertens (Greenwich). 2010. 12:431–438.
Article92. Gupta AK, Cornelissen G, Greenway FL, Dhoopati V, Halberg F, Johnson WD. Abnormalities in circadian blood pressure variability and endothelial function: pragmatic markers for adverse cardiometabolic profiles in asymptomatic obese adults. Cardiovasc Diabetol. 2010. 9:58.
Article93. Diaz KM, Veerabhadrappa P, Kashem MA, Feairheller DL, Sturgeon KM, Williamson ST, et al. Relationship of visit-to-visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertens Res. 2012. 35:55–61.
Article94. Stergiou GS, Parati G. How to best assess blood pressure? The ongoing debate on the clinical value of blood pressure average and variability. Hypertension. 2011. 57:1041–1042.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypertension and Vascular Aging
- Stroke Update: Optimal Blood Pressure Management for Stroke Prevention
- Left ventricular hypertrophy and diastolic function in children and adolescents with essential hypertension
- Blood Pressure Variability and Cardiovascular Risk
- Hypertension and Vascular Disease: Pharmacologic Management of Hypertension