J Korean Assoc Pediatr Surg.
2016 Jun;22(1):14-17. 10.13029/jkaps.2016.22.1.14.
A Pediatric Case of Mixed Acinar-Neuroendocrine Carcinoma
- Affiliations
-
- 1Department of Pediatric Surgery, Seoul National University Children's Hospital, Seoul, Korea. spkhy02@snu.ac.kr
- KMID: 2327656
- DOI: http://doi.org/10.13029/jkaps.2016.22.1.14
Abstract
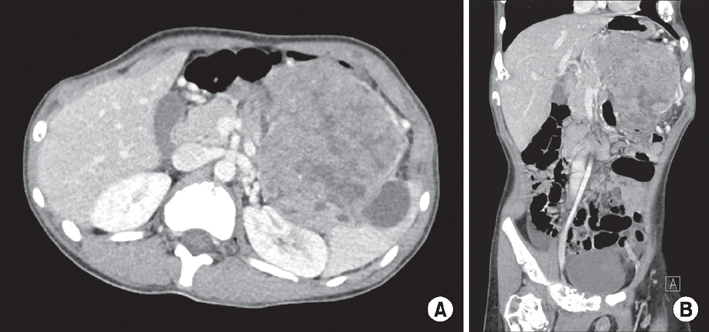
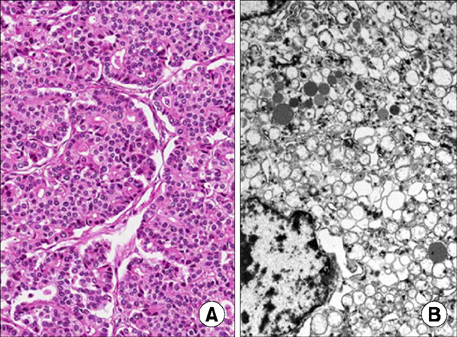
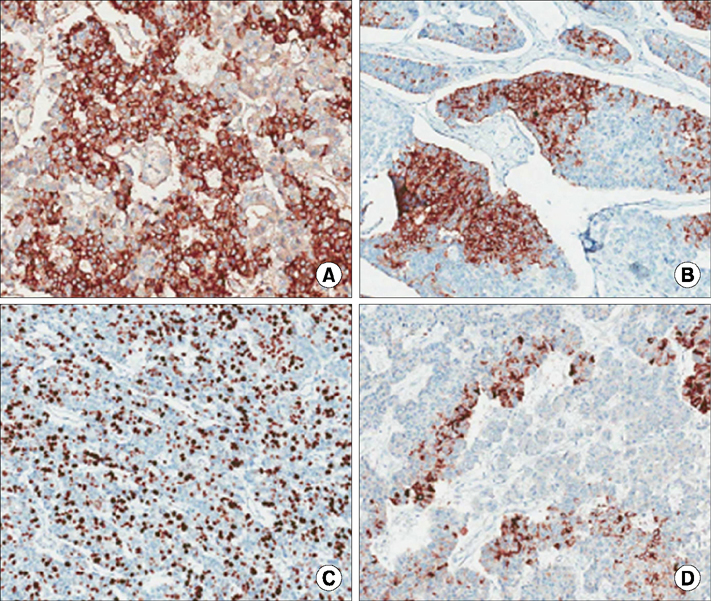
- Mixed acinar-neuroendocrine carcinoma (MANEC) is a malignant pancreatic tumor that rarely occurs in children. It is diagnosed pathologically according to the proportion of neuroendocrine cells present, highlighting the need for surgical biopsy. A 13-year-old boy presented with a 10-cm palpable mass on CT. Surgical resection was performed, and the pathological diagnosis was MANEC. There were no postoperative complications, and the patient was discharged from the hospital 10 days after surgery. He is presently undergoing adjuvant chemotherapy. We reviewed historical MANEC cases published in the English literature. We concluded that pathological analysis of a surgically resected specimen is necessary for an accurate diagnosis of MANEC, and that publication of more cases is needed to determine the optimal management strategy for MANEC.
Keyword
MeSH Terms
Figure
Reference
-
1. Ogbonna OH, Garcon MC, Syrigos KN, Saif MW. Mixed acinarneuroendocrine carcinoma of the pancreas with neuroendocrine predominance. Case Rep Med. 2013; 2013:705092.2. Stelow EB, Shaco-Levy R, Bao F, Garcia J, Klimstra DS. Pancreatic acinar cell carcinomas with prominent ductal differentiation: mixed acinar ductal carcinoma and mixed acinar endocrine ductal carcinoma. Am J Surg Pathol. 2010; 34:510–518.3. Muller CO, Guérin F, Goldzmidt D, Fouquet V, Franchi-Abella S, Fabre M, et al. Pancreatic resections for solid or cystic pancreatic masses in children. J Pediatr Gastroenterol Nutr. 2012; 54:369–373.4. Klimstra DS, Rosai J, Heffess CS. Mixed acinar-endocrine carcinomas of the pancreas. Am J Surg Pathol. 1994; 18:765–778.5. Lee L, Bajor-Dattilo EB, Das K. Metastatic mixed acinar-neuroendocrine carcinoma of the pancreas to the liver: a cytopathology case report with review of the literature. Diagn Cytopathol. 2013; 41:164–170.6. Ohike N, Kosmahl M, Klöppel G. Mixed acinar-endocrine carcinoma of the pancreas. A clinicopathological study and comparison with acinar-cell carcinoma. Virchows Arch. 2004; 445:231–235.7. Ordóñez NG, Balsaver AM, Mackay B. Mucinous islet cell (amphicrine) carcinoma of the pancreas associated with watery diarrhea and hypokalemia syndrome. Hum Pathol. 1988; 19:1458–1461.8. Terada T, Matsunaga Y, Maeta H, Endo K, Horie S, Ohta T. Mixed ductal-endocrine carcinoma of the pancreas presenting as gastrinoma with Zollinger-Ellison syndrome: an autopsy case with a 24-year survival period. Virchows Arch. 1999; 435:606–611.9. Sigel CS, Klimstra DS. Cytomorphologic and immunophenotypical features of acinar cell neoplasms of the pancreas. Cancer Cytopathol. 2013; 121:459–470.10. Wood LD, Klimstra DS. Pathology and genetics of pancreatic neoplasms with acinar differentiation. Semin Diagn Pathol. 2014; 31:491–497.11. Iglesias-Garcia J, Lariño-Noia J, Abdulkader I, Domínguez-Muñoz JE. Rapid on-site evaluation of endoscopic-ultrasound-guided fine-needle aspiration diagnosis of pancreatic masses. World J Gastroenterol. 2014; 20:9451–9457.12. Antoine M, Khitrik-Palchuk M, Saif MW. Long-term survival in a patient with acinar cell carcinoma of pancreas. A case report and review of literature. JOP. 2007; 8:783–789.13. Imamura M, Kimura Y, Ito H, Nobuoka T, Koito K, Sasaki A, et al. Acinar cell carcinoma of the pancreas with intraductal growth: report of a case. Surg Today. 2009; 39:1006–1009.14. Kyriazi MA, Arkadopoulos N, Stafyla VK, Yiallourou AI, Dafnios N, Theodosopoulos T, et al. Mixed acinar-endocrine carcinoma of the pancreas: a case report and review of the literature. Cases J. 2009; 2:6481.15. Shonaka T, Inagaki M, Akabane H, Yanagida N, Shomura H, Yanagawa N, et al. Total pancreatectomy for metachronous mixed acinar-ductal carcinoma in a remnant pancreas. World J Gastroenterol. 2014; 20:11904–11909.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acinar Cell Carcinoma of the Pancreas: A case report
- Mixed acinar-endocrine carcinoma of the pancreas: a case report
- A Case of Mixed Small and Large Cell Neuroendocrine Carcinoma of the Uterine Cervix
- Acinar Cell Carcinoma of the Pancreas: Clinical and Cytomorphologic Characteristics
- Fine Needle Aspiration Cytologic Findings of Pulmonary Neuroendocrine Tumors