J Nutr Health.
2013 Dec;46(6):493-502.
Effect of fermented soybean curd residue (FSCR; SCR-meju) by aspergillus oryzae on the anti-obesity and lipids improvement
- Affiliations
-
- 1Department of Food, Nutrition and Culinary Arts, Keimyung College, Daegu 704-703, Korea.
- 2Center for Nutraceutical and Pharmaceutical Materials, Myongji University, Yongin 449-728, Korea. rheeinae@hanmail.net
- 3Department of Cosmetic Science, Chungwoon University, Hongseong 350-701, Korea.
Abstract
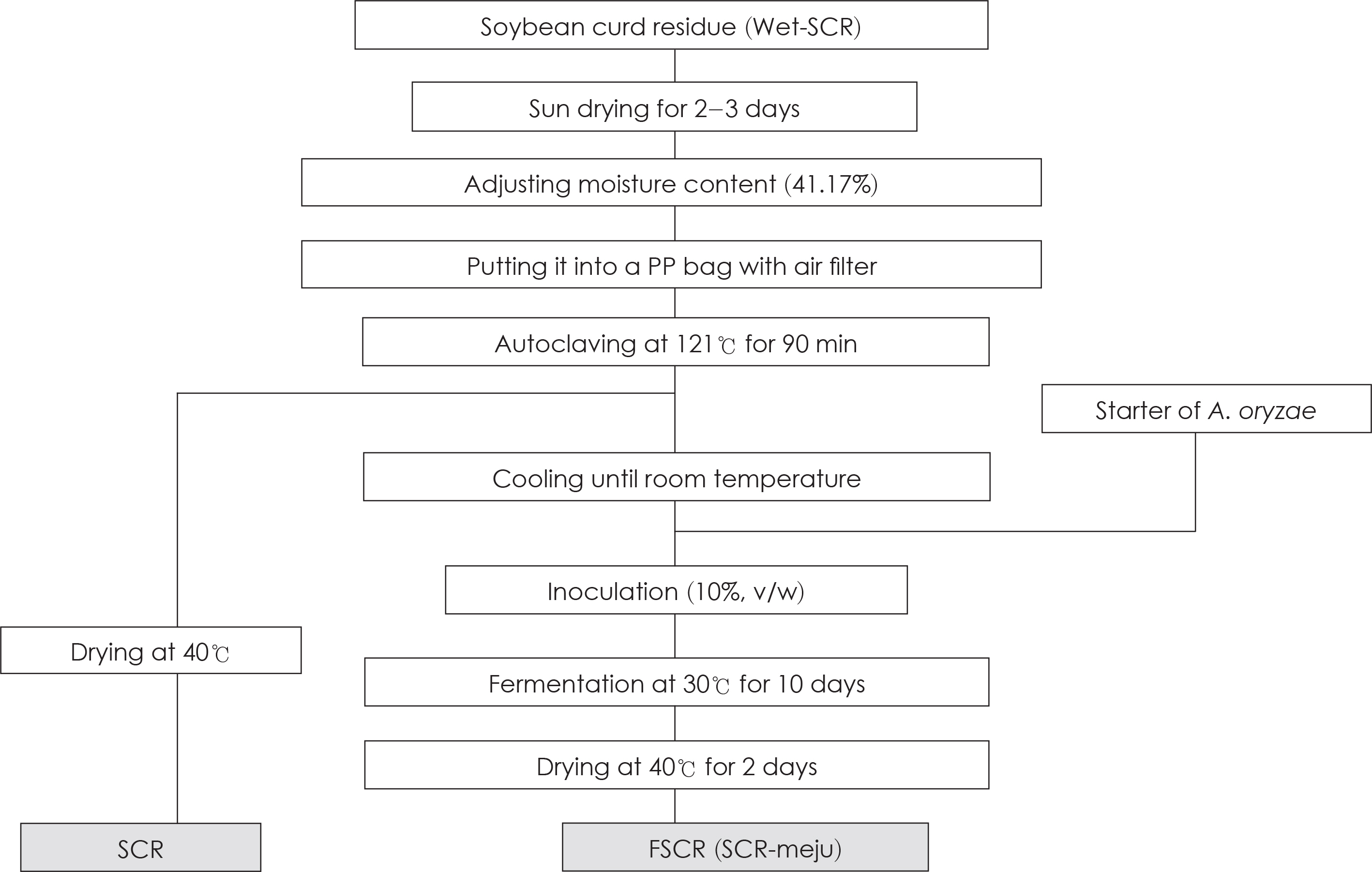
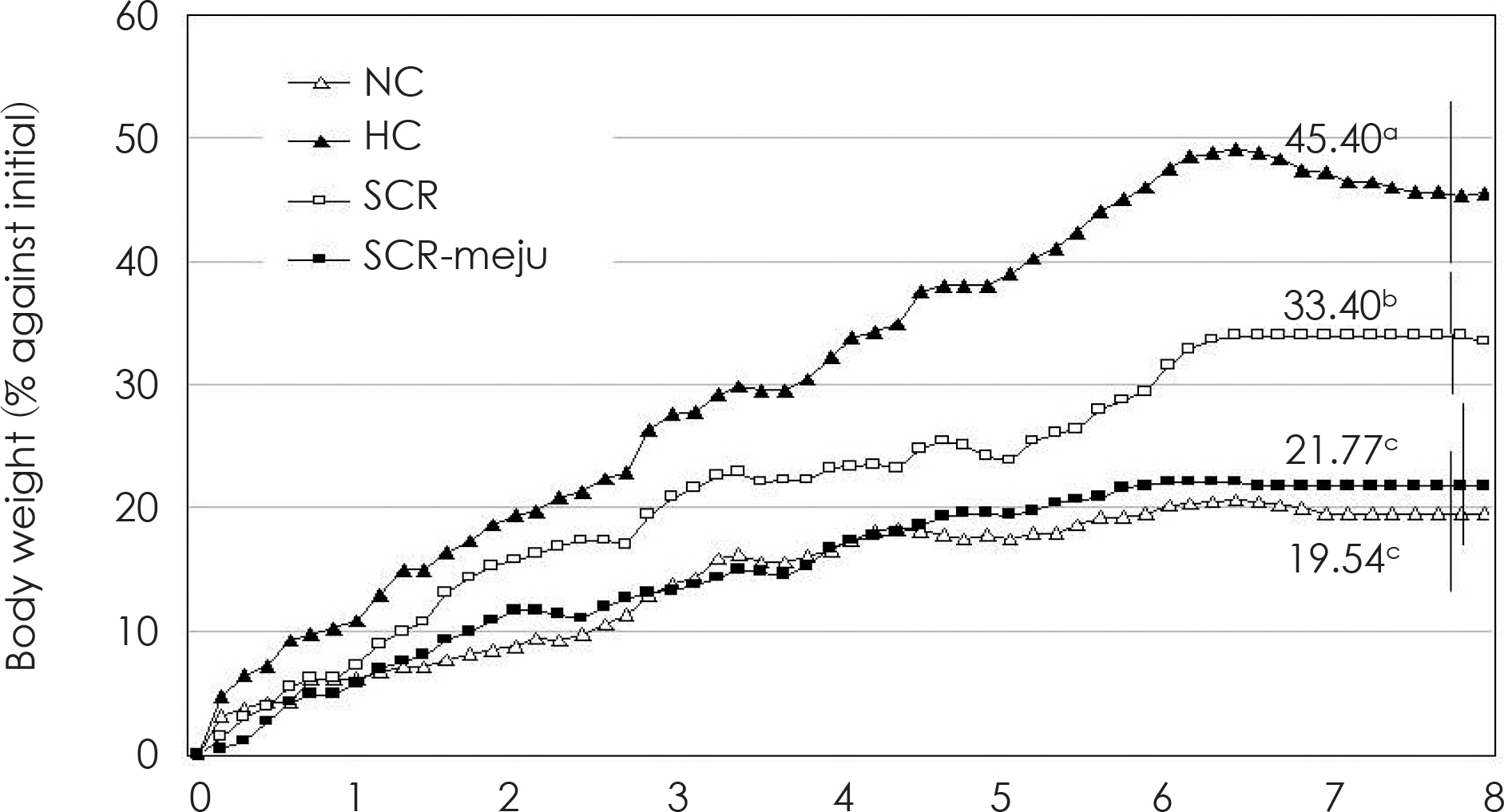
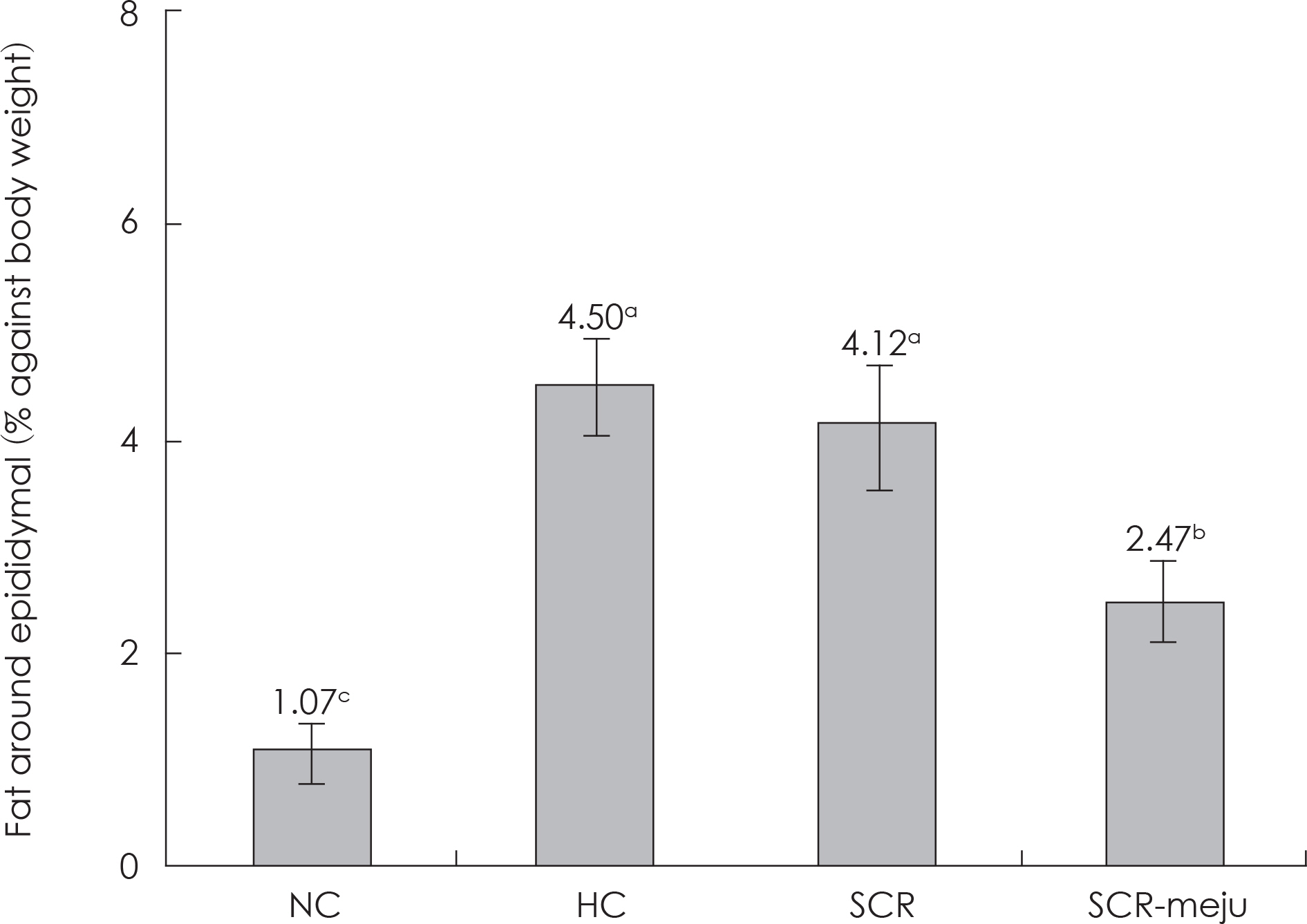
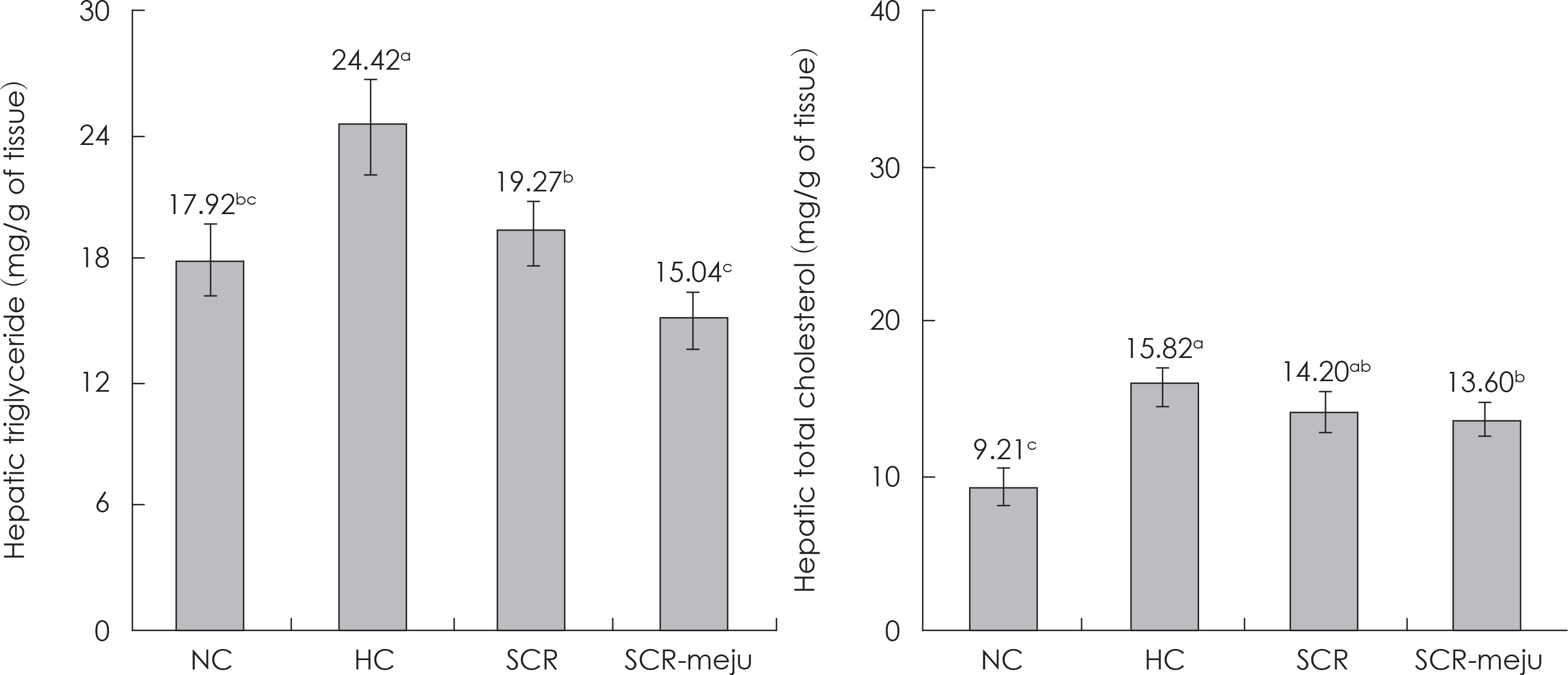
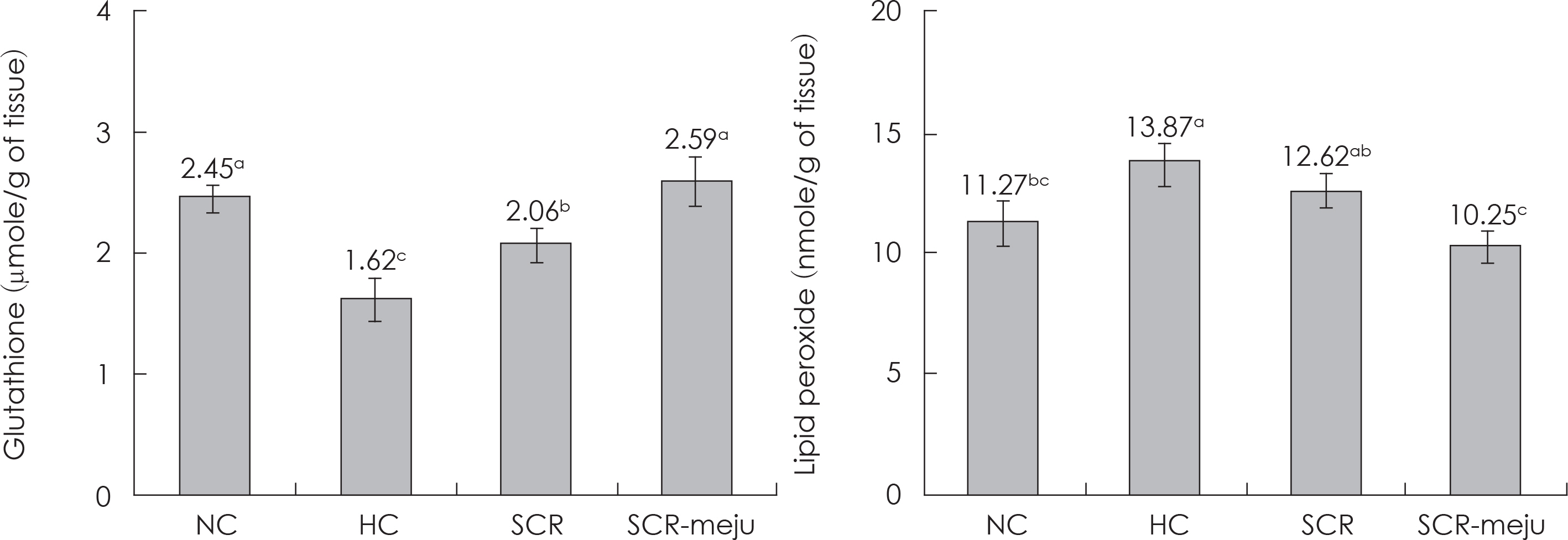
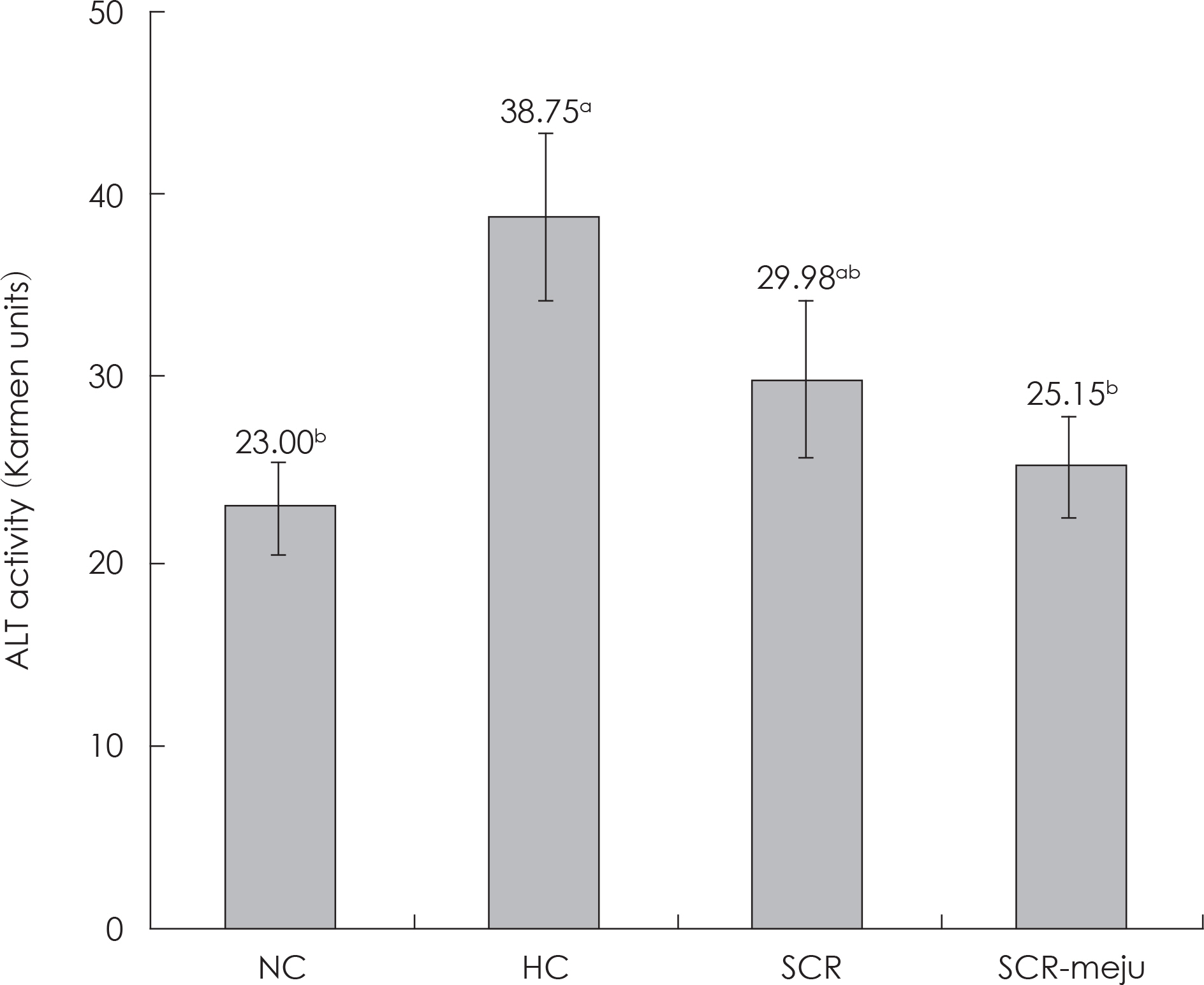
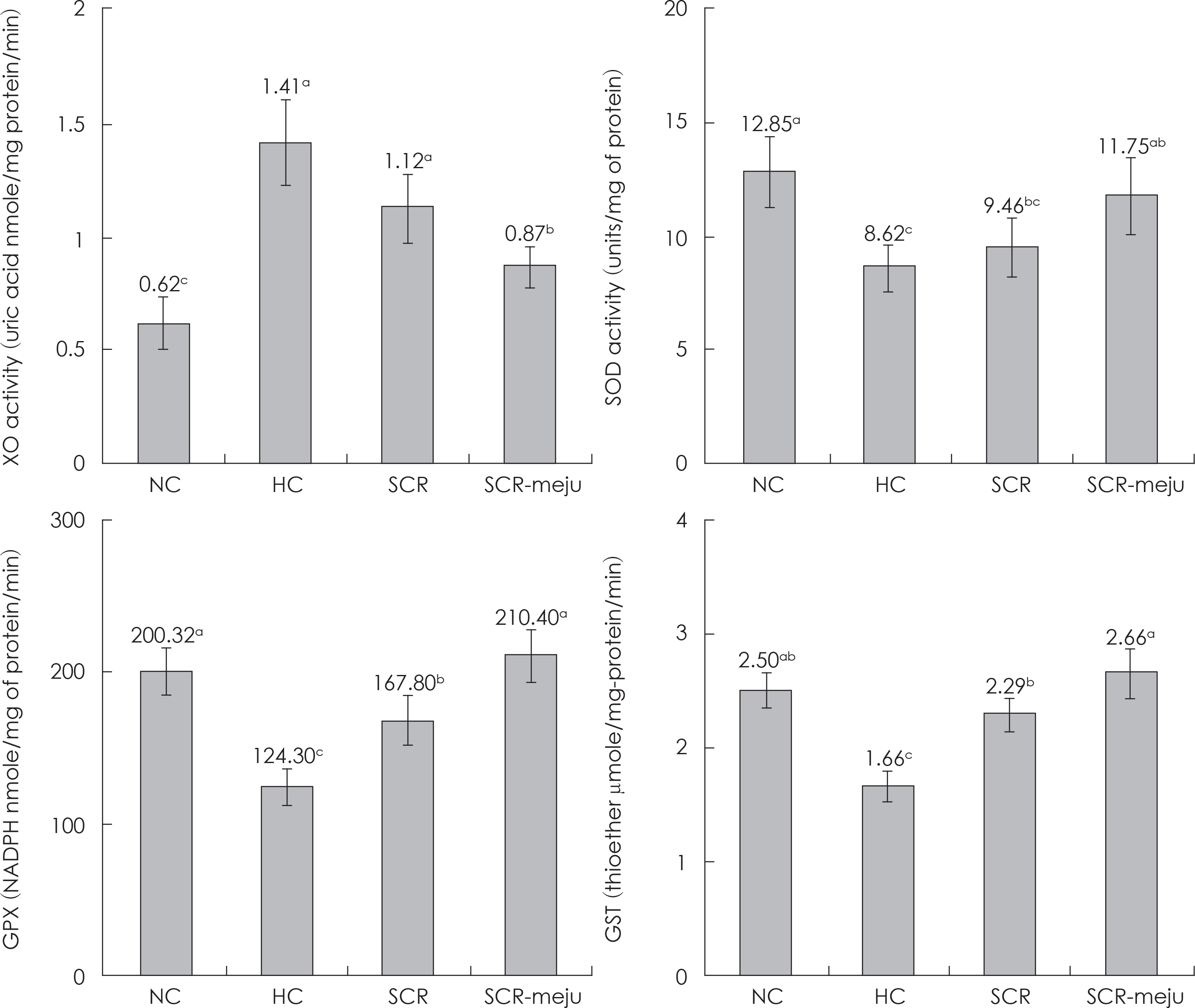
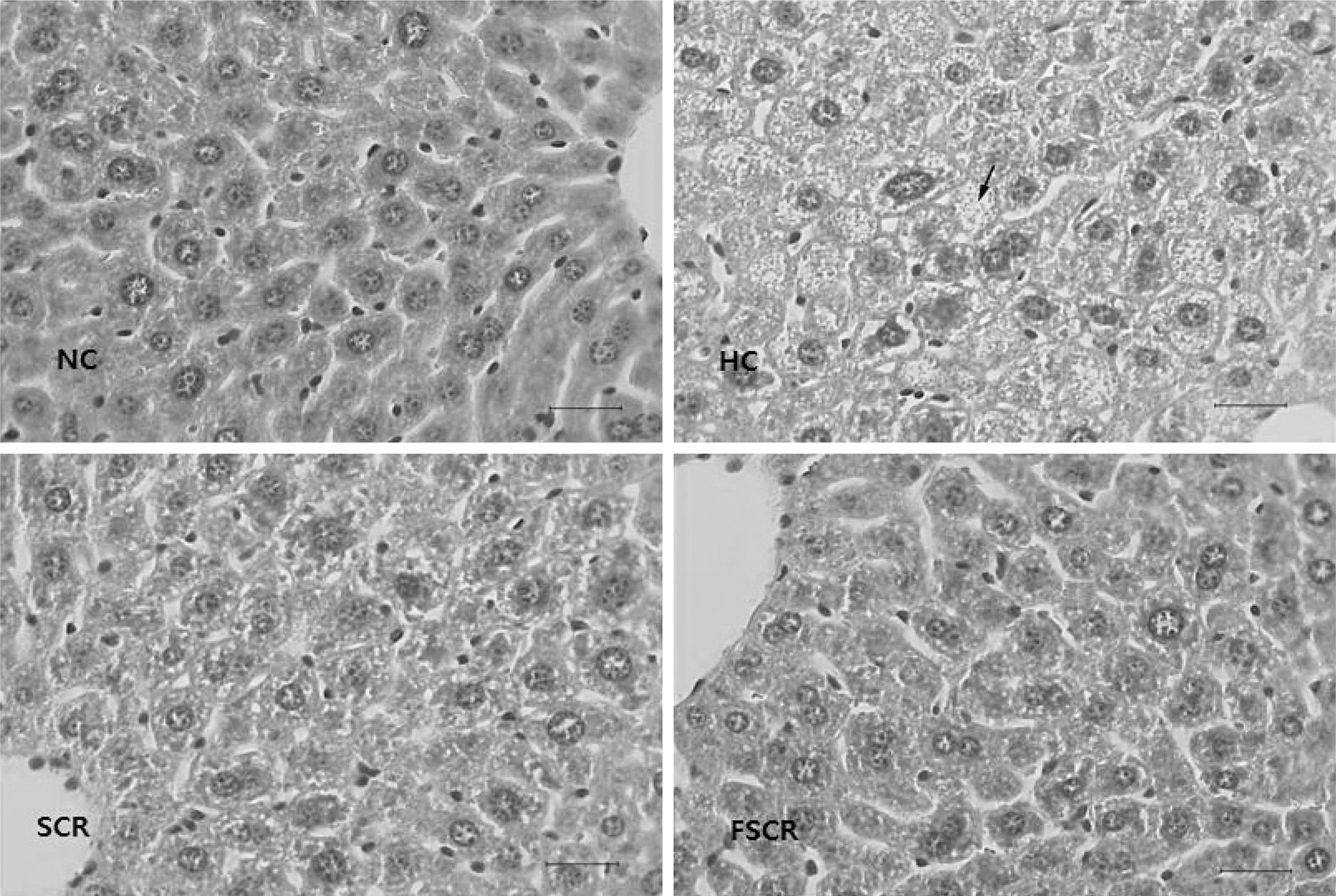
- In this study, we designed to confirm the dietary effect of anti-obesity of fermented soybean curd residue (FSCR; SCR-Meju; Biji-meju) by A. oryzae, which is well known as a Korean traditional meju microbe. We observed that body weight gain, serum and hepatic lipid profile, as well as the activity of ROS generating enzyme and ROS scavenging enzyme in high-fat diet induced obese mice fed experimental diet (SCR and SCR-meju). Body weight gain and epididymal fat weight of HC (high-fat diet control) was markedly higher than that of NC (Normal control). Conversely, body weight gain and epididymal fat weight of the SCR (Biji) and SCR-meju (Biji-meju) group was significantly lower than that of HC; these of the SCR-meju group was lower than that of the SCR group. Furthermore, serum TG and total-cholesterol and LDL-cholesterol contents of SCR and SCR-meju groups were lower than that of HC, and HDL-cholesterol level of the SCR-meju group was significantly higher than that of HC. In conclusion, although precise mechanisms of the antiobese effects of SCR-meju in this study are unknown, the present study provides an experimental evidence that SCR-meju may prevent obesity and obesity related metabolic syndromes, such as hyperlipidemia, hypertension and diabetes, and liver disease by high-fat diet. Nevertheless, further study in this filed will be needed.
Keyword
MeSH Terms
Figure
Reference
-
References
1). Kopelman PG. Obesity as a medical problem. Nature. 2000; 404(6778):635–643.
Article2). Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world–a growing challenge. N Engl J Med. 2007; 356(3):213–215.3). Grundy SM. Multifactorial causation of obesity: implications for prevention. Am J Clin Nutr. 1998; 67(3 Suppl):563S–572S.
Article4). Ferraro KF, Su YP, Gretebeck RJ, Black DR, Badylak SF. Body mass index and disability in adulthood: a 20-year panel study. Am J Public Health. 2002; 92(5):834–840.
Article5). Mukherjee M. Human digestive and metabolic lipases – a brief review. J Mol Catal B Enzym. 2003; 22(5–6):369–376.6). Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004; 114(12):1752–1761.
Article7). Drew BS, Dixon AF, Dixon JB. Obesity management: update on orlistat. Vasc Health Risk Manag. 2007; 3(6):817–821.8). Poston WS, Foreyt JP. Sibutramine and the management of obesity. Expert Opin Pharmacother. 2004; 5(3):633–642.
Article9). Karamadoukis L, Shivashankar GH, Ludeman L, Williams AJ. An unusual complication of treatment with orlistat. Clin Nephrol. 2009; 71(4):430–432.
Article10). Slovacek L, Pavlik V, Slovackova B. The effect of sibutramine therapy on occurrence of depression symptoms among obese patients. Nutr Metab Cardiovasc Dis. 2008; 18(8):e43–e44.
Article11). Birari RB, Bhutani KK. Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discov Today. 2007; 12(19–20):879–889.
Article12). Halford JC, Blundell JE. Pharmacology of appetite suppression. Prog Drug Res. 2000; 54:25–58.
Article13). Redinger RN. Fat storage and the biology of energy expenditure. Transl Res. 2009; 154(2):52–60.
Article14). Madsen L, Petersen RK, Kristiansen K. Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim Biophys Acta. 2005; 1740(2):266–286.
Article15). Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res. 2006; 53(6):482–491.
Article16). Rayalam S, Della-Fera MA, Baile CA. Phytochemicals and regulation of the adipocyte life cycle. J Nutr Biochem. 2008; 19(11):717–726.
Article17). Yang JY, Lee SJ, Park HW, Cha YS. Effect of genistein with car-nitine administration on lipid parameters and obesity in C57Bl/6J mice fed a high-fat diet. J Med Food. 2006; 9(4):459–467.
Article18). Moriyama T, Kishimoto K, Nagai K, Urade R, Ogawa T, Utsumi S, Maruyama N, Maebuchi M. Soybean β-conglycinin diet suppresses serum triglyceride levels in normal and genetically obese mice by induction of β-oxidation, downregulation of fatty acid synthase, and inhibition of triglyceride absorption. Biosci Biotechnol Biochem. 2004; 68(2):352–359.
Article19). Harmon AW, Harp JB. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am J Physiol Cell Physiol. 2001; 280(4):C807–C813.
Article20). Shi M, Yang Y, Guan D, Zhang Y, Zhang Z. Bioactivity of the crude polysaccharides from fermented soybean curd residue by Flam-mulina velutipes. Carbohydr Polym. 2012; 89(4):1268–1276.
Article21). Choi MS, Kim JI, Jeong JB, Lee S, Jeong JN, Jeong HJ, Seo EW, Kim TY, Kwon OJ, Lim JH. Suppressive effects of by-product extracts from soybean on adipocyte differentiation and expression of obesity-related genes in 3T3-L1 adipocytes. J Life Sci. 2011; 21(3):358–367.
Article22). Matsumoto K, Watanabe Y, Yokoyama S. Okara, soybean residue, prevents obesity in a diet-induced murine obesity model. Biosci Biotechnol Biochem. 2007; 71(3):720–727.
Article23). Choi UK, Kim MH, Lee NH, Jeong YS, Hwang YH. Changes in quality characteristics of Meju made with germinated soybean during fermentation. Korean J Food Sci Technol. 2007; 39(3):304–308.24). Lee J, Jeong JY, Cho YS, Park SK, Kim K, Kim MJ, Lee MK. Effect of young Phragmites communis leaves powder on lipid metabolism and erythrocyte antioxidant enzyme activities in high-fat diet fed mice. J Korean Soc Food Sci Nutr. 2010; 39(5):677–683.
Article25). Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transam-inases. Am J Clin Pathol. 1957; 28(1):56–63.
Article26). Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18(6):499–502.
Article27). Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226(1):497–509.
Article28). Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82(1):70–77.
Article29). Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95(2):351–358.
Article30). Stirpe F, Della Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O). J Biol Chem. 1969; 244(14):3855–3863.31). Martin JP Jr, Dailey M, Sugarman E. Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch Biochem Biophys. 1987; 255(2):329–336.
Article32). Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974; 249(22):7130–7139.33). Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70(1):158–169.34). Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193(1):265–275.
Article35). Wu YG, Xia LL, Lin H, Zhou D, Qian H, Lin ST. Prevention of early liver injury by breviscapine in streptozotocin-induced diabetic rats. Planta Med. 2007; 73(5):433–438.
Article36). Ha SK, Chae C. Inducible nitric oxide distribution in the fatty liver of a mouse with high fat diet-induced obesity. Exp Anim. 2010; 59(5):595–604.
Article37). Wang RS, Nakajima T, Honma T. Different change patterns of the isozymes of cytochrome P450 and glutathione S-transferas-es in chemically induced liver damage in rat. Ind Health. 1999; 37(4):440–448.
Article38). Gordon T, Kannel WB, Castelli WP, Dawber TR. Lipoproteins, cardiovascular disease, and death. The Framingham study. Arch Intern Med. 1981; 141(9):1128–1131.
Article39). Yao T, Sato M, Kobayashi Y, Wasa T. Amperometric assays of total and free cholesterols in serum by the combined use of immobilized cholesterol esterase and cholesterol oxidase reactors and peroxidase electrode in a flow injection system. Anal Biochem. 1985; 149(2):387–391.
Article40). Hashim MS, Lincy S, Remya V, Teena M, Anila L. Effect of poly-phenolic compounds from Coriandrum sativum on H2O2-induced oxidative stress in human lymphocytes. Food Chem. 2005; 92(4):653–660.41). Lee SI, Kim JW, Lee YK, Yang SH, Lee IA, Suh JW, Kim SD. Protective effect of Monascus pilosus mycelial extract on hepatic damage in high-fat diet induced-obese rats. J Korean Soc Appl Biol Chem. 2011; 54(3):206–213.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of Nonaflatoxigenic Aspergillus flavus/oryzae Strains Isolated from Korean Traditional Soybean Meju
- Aspergillus Associated with Meju, a Fermented Soybean Starting Material for Traditional Soy Sauce and Soybean Paste in Korea
- Study on Aflatoxins in Korean Fermented Foodstuffs
- Analysis of the MAT1-1 and MAT1-2 Gene Ratio in Black Koji Molds Isolated from Meju
- Soluble extract of soybean fermented with Aspergillus oryzae GB107 inhibits fat accumulation in cultured 3T3-L1 adipocytes