Nutr Res Pract.
2015 Aug;9(4):439-444. 10.4162/nrp.2015.9.4.439.
Soluble extract of soybean fermented with Aspergillus oryzae GB107 inhibits fat accumulation in cultured 3T3-L1 adipocytes
- Affiliations
-
- 1Laboratory of Animal Physiology, Graduate School of Agricultural Science, Tohoku University, Aoba-ku, Sendai 981-8555, Japan. sanggun_roh@bios.tohoku.ac.jp
- 2Faculty of Agriculture, Shinshu University, Nagano-ken 399-4598, Japan.
- 3Genebiotech Co., Seoul, 137-787 Korea.
- 4Department of Animal Science, North Carolina State University, Raleigh, NC 27695, USA.
- KMID: 2313863
- DOI: http://doi.org/10.4162/nrp.2015.9.4.439
Abstract
- BACKGROUND/OBJECTIVES
This study was conducted to investigate the effects of fermented soybean (FS) extract on adipocyte differentiation and fat accumulation using cultured 3T3-L1 adipocytes.
MATERIALS/METHODS
3T3-L1 adipocytes were treated with FS and nonfermented soybean (NFS) extract during differentiation for 10 days in vitro. Oil red O staining was performed and glycerol-3-phosphate dehydrogenase (GPDH) activity was measured for analysis of fat accumulation. Expressions of adipogenic genes were measured.
RESULTS
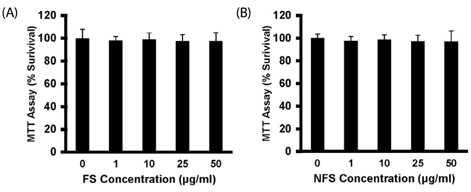
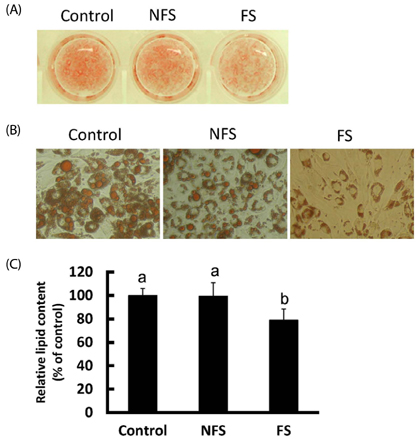
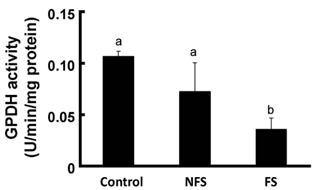
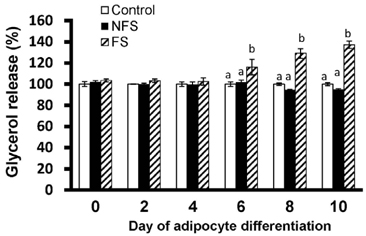
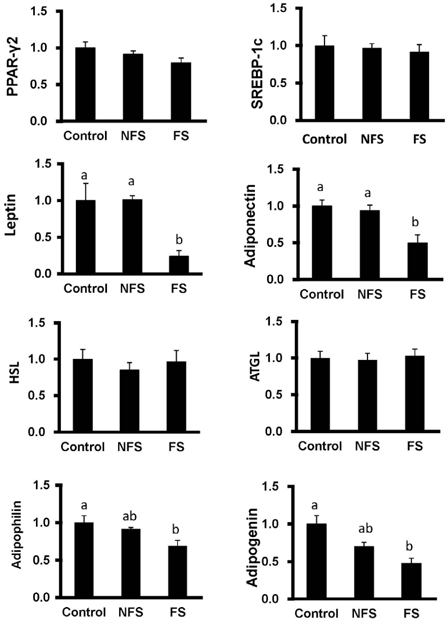
Soluble extract of soybean fermented with Aspergillus oryzae GB107 contained higher levels of low-molecular-weight protein than conventional soybean protein did. FS extract (50 microg/ml) inhibited adipocyte differentiation and fat accumulation during differentiation of 3T3-L1 preadipocytes for 10 days in vitro. Significantly lower GPDH activity was observed in differentiated adipocytes treated with the FS extract than those treated with NFS extract. Treatment with FS extract resulted in decreased expression levels of leptin, adiponectin, and adipogenin genes, which are associated with adipogenesis.
CONCLUSIONS
This report is the first to demonstrate that the water-soluble extract from FS inhibits fat accumulation and lipid storage in 3T3-L1 adipocytes. Thus, the soybean extract fermented with A. oryzae GB107 could be used to control lipid accumulation in adipocytes.
Keyword
MeSH Terms
Figure
Reference
-
1. Hong KJ, Lee CH, Kim SW. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J Med Food. 2004; 7:430–435.
Article2. Kim SW, van Heugten E, Ji F, Lee CH, Mateo RD. Fermented soybean meal as a vegetable protein source for nursery pigs: I. Effects on growth performance of nursery pigs. J Anim Sci. 2010; 88:214–224.
Article3. Roh SG, Carroll JA, Kim SW. Effects of fermented soybean meal on innate immunity-related gene expressions in nursery pigs acutely challenged with lipopolysaccharides. Anim Sci J. Forthcoming 2014.
Article4. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998; 395:763–770.
Article5. Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998; 78:783–809.
Article6. Roh SG, Hishikawa D, Hong YH, Sasaki S. Control of adipogenesis in ruminants. Anim Sci J. 2006; 77:472–477.
Article7. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994; 372:425–432.
Article8. Sethi JK, Xu H, Uysal KT, Wiesbrock SM, Scheja L, Hotamisligil GS. Characterisation of receptor-specific TNFalpha functions in adipocyte cell lines lacking type 1 and 2 TNF receptors. FEBS Lett. 2000; 469:77–82.
Article9. Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003; 423:762–769.
Article10. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993; 259:87–91.
Article11. Roh SG, Song SH, Choi KC, Katoh K, Wittamer V, Parmentier M, Sasaki S. Chemerin--a new adipokine that modulates adipogenesis via its own receptor. Biochem Biophys Res Commun. 2007; 362:1013–1018.
Article12. Suzuki Y, Hong YH, Song SH, Ardiyanti A, Kato D, So KH, Katoh K, Roh SG. The regulation of chemerin and CMKLR1 genes expression by TNF-α, adiponectin, and chemerin analog in bovine differentiated adipocytes. Asian-Australas J Anim Sci. 2012; 25:1316–1321.
Article13. Suzuki Y, Song SH, Sato K, So KH, Ardiyanti A, Kitayama S, Hong YH, Lee SD, Choi KC, Hagino A, Katoh K, Roh SG. Chemerin analog regulates energy metabolism in sheep. Anim Sci J. 2012; 83:263–267.
Article14. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994; 79:1147–1156.
Article15. Kwon DY, Daily JW 3rd, Kim HJ, Park S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res. 2010; 30:1–13.
Article16. Hong YH, Hishikawa D, Miyahara H, Tsuzuki H, Nishimura Y, Gotoh C, Choi KC, Hokari Y, Takagi Y, Lee HG, Cho KK, Roh SG, Sasaki S. Up-regulation of adipogenin, an adipocyte plasma transmembrane protein, during adipogenesis. Mol Cell Biochem. 2005; 276:133–141.
Article17. Kim HK, Kim JN, Han SN, Nam JH, Na HN, Ha TJ. Black soybean anthocyanins inhibit adipocyte differentiation in 3T3-L1 cells. Nutr Res. 2012; 32:770–777.
Article18. Choi KC, Shrestha YB, Roh SG, Hishikawa D, Kuno M, Tsuzuki H, Hong YH, Sasaki S. The role of phosphatidylinositol 3-kinase and mitogenic activated protein kinase on the differentiation of ovine preadipocytes. Asian-Australas J Anim Sci. 2003; 16:1199–1204.
Article19. Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, Katoh K, Roh SG, Sasaki S. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005; 146:5092–5099.
Article20. Wise LS, Green H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J Biol Chem. 1979; 254:273–275.
Article21. Gonzalez de Mejia E, Martinez-Villaluenga C, Roman M, Bringe NA. Fatty acid synthase and in vitro adipogenic response of human adipocytes inhibited by α and α' subunits of soybean β-conglycinin hydrolysates. Food Chem. 2010; 119:1571–1577.
Article22. Martinez-Villaluenga C, Bringe NA, Berhow MA, Gonzalez de Mejia E. β-Conglycinin embeds active peptides that inhibit lipid accumulation in 3T3-L1 adipocytes in vitro. J Agric Food Chem. 2008; 56:10533–10543.
Article23. Lovati MR, Manzoni C, Canavesi A, Sirtori M, Vaccarino V, Marchi M, Gaddi G, Sirtori CR. Soybean protein diet increases low density lipoprotein receptor activity in mononuclear cells from hypercholesterolemic patients. J Clin Invest. 1987; 80:1498–1502.
Article24. Oakenfull DG, Topping DL. Saponins and plasma cholesterol. Atherosclerosis. 1983; 48:301–303.
Article25. Sidhu GS, Oakenfull DG. A mechanism for the hypocholesterolaemic activity of saponins. Br J Nutr. 1986; 55:643–649.
Article26. Sugano M, Goto S, Yamada Y, Yoshida K, Hashimoto Y, Matsuo T, Kimoto M. Cholesterol-lowering activity of various undigested fractions of soybean protein in rats. J Nutr. 1990; 120:977–985.
Article27. Kwon DY, Hong SM, Ahn IS, Kim MJ, Yang HJ, Park S. Isoflavonoids and peptides from meju, long-term fermented soybeans, increase insulin sensitivity and exert insulinotropic effects in vitro. Nutrition. 2011; 27:244–252.
Article28. Iwai K, Nakaya N, Kawasaki Y, Matsue H. Inhibitory effect of natto, a kind of fermented soybeans, on LDL oxidation in vitro. J Agric Food Chem. 2002; 50:3592–3596.
Article29. Iwai K, Nakaya N, Kawasaki Y, Matsue H. Antioxidative functions of natto, a kind of fermented soybeans: effect on LDL oxidation and lipid metabolism in cholesterol-fed rats. J Agric Food Chem. 2002; 50:3597–3601.
Article30. de Mejia EG, Dia VP. Lunasin and lunasin-like peptides inhibit inflammation through suppression of NF-kappaB pathway in the macrophage. Peptides. 2009; 30:2388–2398.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Aspergillus oryzae S-03 Produces Gingipain Inhibitors as a Virulence Factor for Porphyromonas gingivalis