J Nutr Health.
2014 Dec;47(6):394-402. 10.4163/jnh.2014.47.6.394.
Anti-diabetic effects of aqueous and ethanol extract of Dendropanax morbifera Leveille in streptozotocin-induced diabetes model
- Affiliations
-
- 1Department of Food Science and Nutrition, Pusan National University, Pusan 609-735, Korea. hokryu@pusan.ac.kr
- 2Department of Biomaterials Science, College of Natural Resources & Life Science, Pusan National University, Miryang 627-706, Korea.
- KMID: 2327094
- DOI: http://doi.org/10.4163/jnh.2014.47.6.394
Abstract
- PURPOSE
Dendropanax morifera Leveille(DML) exhibits diverse biological and pharmacological activities, including anti-oxidative effect, anti-cancer activity, hepatoprotection, immunological stimulation, and bone regeneration. As part of the identification for novel functions of DML, we investigated the therapeutic effects of DML on diabetes induced by streptozotocine (STZ) treatment.
METHODS
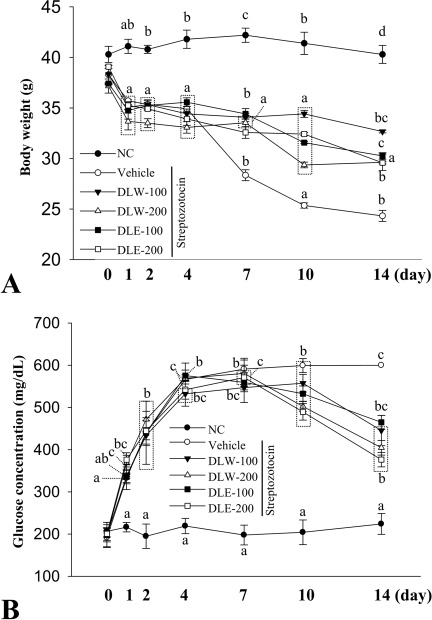
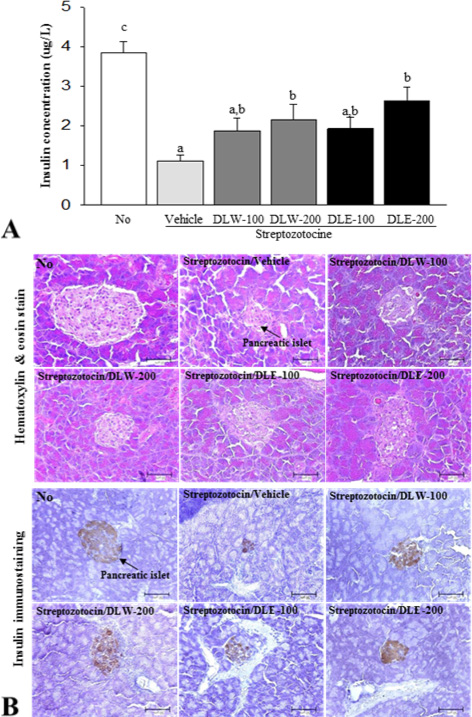
First, the four extracts including the water extract of leaf (DLW), the ethanol extract of leaf (DLE), the water extract of stem (DSW), and the ethanol extract of stem (DSE) were collected from the leaf and stem of DML using a hot water and ethanol solvent. Alterations in body weight, glucose concentration, insulin level, and pancreatic islet structure were investigated in diabetic mice after treatment with extracts of DML for 2 weeks.
RESULTS
Among four extracts, the highest level of total polyphenols and total flavonoids was detected in DLW, while the lowest level of these was measured in DSE. The radical scavenging activity was also higher in DLW than in the other three extracts at the concentration of 25-100 microg/mL, although this activity was maintained at a constant level in all groups at the concentration of 500 microg/mL. Based on the results of anti-oxidant activity, DLW and DLE were selected for examination of anti-diabetic effects in a diabetes model. Body weight was gradually decreased in all STZ treated groups compared with the No treated group. However, four STZ/DML treated groups maintained a high level of body weight during 7-14 days, while the STZ/vehicle treated group showed a gradual decrease of body weight during the same period. Also, a significant decrease or increase in the concentration of glucose and insulin in the blood of the diabetes model was detected in a subset of groups, although the highest increase was detected in the STZ/DLE-200 treated group. In addition, the histological structure of pancreatic islet was significantly recovered after treatment with DLW and DLE.
CONCLUSION
These results suggest that DLW and DLE may contribute to attenuation of clinical symptoms of diabetes as well as prevent the destruction of pancreatic beta-cells in STZ-induced diabetes mice.
MeSH Terms
Figure
Reference
-
1. Bernart MW, Cardellina JH 2nd, Balaschak MS, Alexander MR, Shoemaker RH, Boyd MR. Cytotoxic falcarinol oxylipins from Dendropanax arboreus. J Nat Prod. 1996; 59(8):748–753.
Article2. Jeong BS, Jo JS, Pyo BS, Hwang B. Studies on the distribution of Dendropanax morbifera and component analysis of the golden lacquer. Korean J Biotechnol Bioeng. 1995; 10(4):393–400.3. Kong YT, Kang IA. Properties of paint film of "Hwangchil" - an ancient Korean natural golden varnish. J Korea For Energy. 1993; 13(1):1–6.4. Bae KH, Kim JA, Choi YE. Induction and in vitro proliferation of adventitious roots in Dendropanax morbifera. J Plant Biotechnol. 2009; 36(2):163–169.
Article5. Moon CG. Antioxidant activity of Dendropanax morbifera Lev [dissertation]. Gimhae: Inje University;2007.6. Chung IM, Kim MY, Park SD, Park WH, Moon HI. In vitro evaluation of the antiplasmodial activity of Dendropanax morbifera against chloroquine-sensitive strains of Plasmodium falciparum. Phytother Res. 2009; 23(11):1634–1637.7. Park BY, Min BS, Oh SR, Kim JH, Kim TJ, Kim DH, Bae KH, Lee HK. Isolation and anticomplement activity of compounds from Dendropanax morbifera. J Ethnopharmacol. 2004; 90(2-3):403–408.
Article8. Yang JW. Protective effect of hot water extract of Dendropanax morbifera Lev on ethanol-induced liver damage [dissertation]. Seongnam: CHA University;2010.9. Yu HY. Anti-cancer and anti-inflammatory activities of natural compounds isolated from hyul-tong-ryung and Dendropanax morbifera [dissertation]. Busan: Dong-A University;2010.10. Chung IM, Kim MY, Park WH, Moon HI. Antiatherogenic activity of Dendropanax morbifera essential oil in rats. Pharmazie. 2009; 64(8):547–549.11. Beak WB. Biological activity studies of Korea specialties Dendropanax morbifera [dissertation]. Seoul: Kyunghee University;2003.12. Li JC, Shen XF, Meng XL. A traditional Chinese medicine JiuHuangLian (Rhizoma coptidis steamed with rice wine) reduces oxidative stress injury in type 2 diabetic rats. Food Chem Toxicol. 2013; 59:222–229.
Article13. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005; 54(6):1615–1625.14. Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001; 280(5):E685–E694.
Article15. McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002; 51(1):7–18.16. Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003; 552(Pt 2):335–344.
Article17. Sellamuthu PS, Arulselvan P, Fakurazi S, Kandasamy M. Beneficial effects of mangiferin isolated from Salacia chinensis on biochemical and hematological parameters in rats with streptozotocin-induced diabetes. Pak J Pharm Sci. 2014; 27(1):161–167.18. Abo-elmatty DM, Essawy SS, Badr JM, Sterner O. Antioxidant and anti-inflammatory effects of Urtica pilulifera extracts in type 2 diabetic rats. J Ethnopharmacol. 2013; 145(1):269–277.19. Soon YY, Tan BK. Evaluation of the hypoglycemic and anti-oxidant activities of Morinda officinalis in streptozotocin-induced diabetic rats. Singapore Med J. 2002; 43(2):077–085.20. Moon HI. Antidiabetic effects of dendropanoxide from leaves of Dendropanax morbifera Leveille in normal and streptozotocin-induced diabetic rats. Hum Exp Toxicol. 2011; 30(8):870–875.
Article21. Hatano T, Edamatsu R, Hiramatsu M, Mori A, Fujita Y, Yasuhara T, Yoshida T, Okuda T. Effects of the interaction of tannins with coexisting substances. VI. : effects of tannins and related polyphenols on superoxide anion radical, and on 1, 1-diphenyl-2-picrylhydrazyl radical. Chem Pharm Bull. 1989; 37(8):2016–2021.22. Folin O, Denis W. On phosphotungstic-phosphomolybdic compounds as color reagents. J Biol Chem. 1912; 12:239–243.
Article23. Isla MI, Nieva Moreno MI, Sampietro AR, Vattuone MA. Antioxidant activity of Argentine propolis extracts. J Ethnopharmacol. 2001; 76(2):165–170.
Article24. Zhang XH, Choi SK, Seo JS. Effect of dietary grape pomace on lipid oxidation and related enzyme activities in rats fed high fat diet. Korean J Nutr. 2009; 42(5):415–422.
Article25. Park SN. Skin aging and antioxidant. J Soc Cosmet Sci Korea. 1997; 23(1):75–132.26. Jung MJ, Heo SI, Wang MH. Comparative studies for component analysis in acorn powders from Korea and China. Korean J Pharmacogn. 2007; 38(1):90–94.27. Kwon GJ, Choi DS, Wang MH. Biological activities of hot water extracts from Euonymus alatus leaf. Korean J Food Sci Technol. 2007; 39(5):569–574.28. Kim SM, Jeon JS, Kang SW, Kim WR, Lee KD, Um BH. Composition analysis and antioxidant activity of Ojuk (Phyllostachys nigra Munro) leaf tea and shoot tea. J Appl Biol Chem. 2012; 55(2):95–101.
Article29. Lee SH, Lee YM, Lee HS, Kim DK. Anti-oxidative and anti-hyperglycemia effects of Triticum aestivum wheat sprout water extracts on the streptozotocin-induced diabetic mice. Korean J Pharmacogn. 2009; 40(4):408–414.30. Yoon JA, Kim JJ, Song BC. Effects of Opuntia ficus-indica complexes on blood glucose and pancreatic islets histology in streptozotocin-induced diabetic rats. J East Asian Soc Diet Life. 2012; 22(3):334–340.31. Watanabe H, Sumi S, Urushihata T, Kitamura Y, Iwasaki S, Xu G, Yano S, Nio Y, Tamura K. Immunohistochemical studies on vascular endothelial growth factor and platelet endothelial cell adhesion molecule-1/CD-31 in islet transplantation. Pancreas. 2000; 21(2):165–173.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-diabetic effects of aqueous extract of Dendropanax morbifera Lev. leaves in streptozotocin-induced diabetic Sprague-Dawley rats
- Antimicrobial, Antioxidant and Cytotoxic Activities of Dendropanax morbifera Léveille extract for mouthwash and denture cleaning solution
- Induction of Hepatocellular Carcinoma Cell Cycle Arrest and Apoptosis by Dendropanax morbifera Leveille Leaf Extract via the PI3K/AKT/mTOR Pathway
- Hypolipidemic and hypoglycemic effects of Orostachys japonicus A. Berger extracts in streptozotocin-induced diabetic rats
- Effects of Fractions of Benincasa hispida on Antioxidative Status in Streptozotocin Induced Diabetic Rats