Nutr Res Pract.
2011 Aug;5(4):301-307.
Hypolipidemic and hypoglycemic effects of Orostachys japonicus A. Berger extracts in streptozotocin-induced diabetic rats
- Affiliations
-
- 1Department of Food and Nutrition, Institute of Agriculture and Life Science, Gyeongsang National University, 501 Jinjudaero, Jinju, Gyungnam 660-701, Korea. snakju@gnu.ac.kr
Abstract
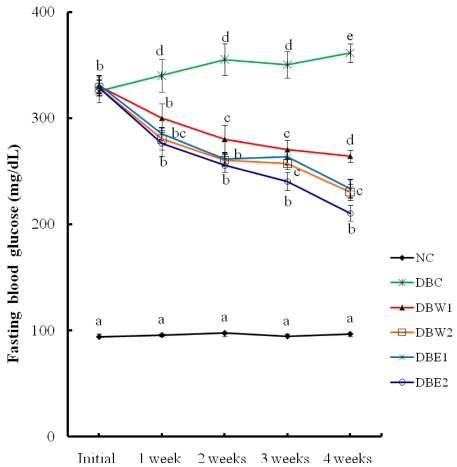
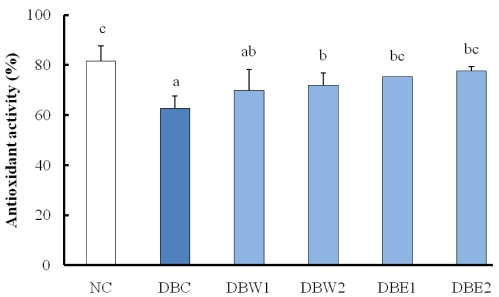
- The hypolipidemic and hypoglycemic effects of two dietary dosages (0.1% and 0.5%) of water and 80% ethanol extracts from hot-air dried Orostachys japonicus A. Berger were evaluated in the serum and organ tissues of streptozotocin-induced diabetic rats. The STZ-induced diabetic groups supplemented with the O. japonicus extracts showed significantly higher body weight compared to a diabetic control group at the end of experiment. The extracts exhibited substantial hypoglycemic effects by significant reductions of fasting blood glucose levels at all time points tested compared to the initial stage before treatment of the extracts. Declines of serum and hepatic triglyceride levels were greater than declines of total cholesterol in the groups treated with the 0.5% O. japonicus extract (DBW2 and DBE2) when compared to the DBC group. Hepatic glycogen content was higher in the groups treated with O. japonicus extract, while lipid peroxide content was decreased in these treated groups compared to the DBC group. Hepatic antioxidant activity was significantly increased in the groups supplemented with the O. japonicus ethanol extract. The hypolipidemic and hypoglycemic effects of the O. japonicus ethanol extract were significantly greater than the effects of the water extract. Based on this study, it seems that O. japonicus ethanol extract, due to its higher phenolic and flavonoid components than the water extract, may control blood glucose and alleviate hyperlipidemia in diabetes.
Keyword
MeSH Terms
Figure
Reference
-
1. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003; 26:S5–S20. PMID: 12502614.2. Chung HR, Pérez-Escamilla R. Risk factors of type 2 diabetes among Korean adults: The 2001 Korean national health and nutrition examination survey. Nutr Res Pract. 2009; 3:286–294. PMID: 20098581.
Article3. Carmena R. Type 2 diabetes, dyslipidemia, and vascular risk: Rationale and evidence for correcting the lipid imbalance. Am Heart J. 2005; 150:859–870. PMID: 16290951.
Article4. Ko M, Kim MT, Nam JJ. Assessing risk factors of coronary heart disease and its risk prediction among Korean adults: The 2001 Korea national health and nutrition examination survey. Int J Cardiol. 2006; 110:184–190. PMID: 16412525.
Article5. Park SY, Han JS. Effects of web-based nutrition counseling on nutrient intake and blood glucose in Type II diabetic patients. J Korean Soc Food Sci Nutr. 2005; 34:1398–1406.6. Yoon JA, Son YS. Effects of Opuntia ficus-indica complexes B (OCB) on blood glucose and lipid metabolism in streptozotocin-induced diabetic rats. Korean J Food Nutr. 2009; 22:48–56.7. Han HK, Yoon SJ, Kim GH. Effects of compositae plants on plasma glucose and lipid level in streptozotocin induced diabetic rats. J Korean Soc Food Sci Nutr. 2009; 38:674–682.
Article8. Kim JY, Park JY, Lee KU. Diabetes and traditional medicine: effect of several traditional drugs on the plasma glucose levels in streptozotocin-induced diabetic rats. J Korean Diabetes Assoc. 1994; 18:377–381.9. Park MJ, Han JS. Antioxidant activity of medicinal plant extracts used as folk remedies by diabetic patients. J Food Sci Nutr. 2004; 9:167–173.
Article10. Kim JK. Illustrated Natural Drugs. 1984. Seoul: Namsandang Publishing Co.;p. 447.11. Park HJ, Young HS, Kim JO, Rhee SH, Choi JS. A study on the chemical constituents of Orostachys japonicus A. Berger. Korean J Pharmacogn. 1991; 22:78–84.12. Park HJ, Lim SC, Lee MS, Young HS. Triterpene and steroids from Orostachys japonicus. Korean J Pharmacogn. 1994; 25:20–23.13. Park HJ, Moon SH, Park KY, Choi JS, Chung HY, Young HS, Suh SS. Antimutagenic effect of Orostachys japonicus. Yakhak Hoeji. 1991; 35:253–257.14. Park HJ, Young HS, Park KY, Rhee SH, Chung HY, Choi JS. Flavonoids from the whole plants of Orostachys japonicus. Arch Pharm Res. 1991; 14:167–171.15. Lee JH, Lee SJ, Park S, Kim HK, Jeong WY, Choi JY, Sung NJ, Lee WS, Lim CS, Kim GS, Shin SC. Characterisation of flavonoids in Orostachys japonicus A. Berger using HPLC-MS/MS: Contribution to the overall antioxidant effect. Food Chem. 2011; 124:1627–1633.16. Lee SJ, Seo JK, Shin JH, Lee HJ, Sung NJ. Antioxidant activity of wa-song (Orostachys japonicus A. Berger) according to drying methods. J Korean Soc Food Sci Nutr. 2008; 37:605–611.17. Lee SJ, Cha JY, Shin JH, Chung MJ, Sung NJ. Antioxidant effect of wa-song (Orostachys japonicus A. Berger) extracts on edible oil and fat. J Life Sci. 2008; 18:1106–1114.18. Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003; 135C:357–364. PMID: 12927910.
Article19. Kim JH, Kang MJ, Choi HN, Jeong SM, Lee YM, Kim JI. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr Res Pract. 2011; 5:107–111. PMID: 21556223.
Article20. Lee SJ, Song EJ, Lee SY, Kim KBWR, Kim SJ, Yoon SY, Lee CJ, Ahn DH. Antioxidant activity of leaf, stem and root extracts from Orostachys japonicus and their heat and pH stabilities. J Korean Soc Food Sci Nutr. 2009; 38:1571–1579.21. Verspohl EJ. Recommended testing in diabetes research. Plant Med. 2002; 68:581–590.
Article22. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502. PMID: 4337382.
Article23. Haglund O, Loustarinen R, Wallin R, Wibell I, Saldeen T. The effect of oil on triglycerides, cholesterol, fibrinogen and malondialdehyde in mand supplemented with vitamin. Eur J Nutr. 1991; 121:165–172.24. Kang SM, Shim JY, Hwang SJ, Hong S, Jang HE, Park MH. Effects of Saengshik supplementation on health improvement in diet-induced hypercholesterolemic rats. J Korean Soc Food Sci Nutr. 2003; 32:906–912.25. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509. PMID: 13428781.
Article26. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275. PMID: 14907713.
Article27. Carroll NV, Longley RW, Roe JH. The determination of glycogen in liver and muscle by use of anthrone reagent. J Biol Chem. 1956; 220:583–593. PMID: 13331917.
Article28. Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978; 86:271–278. PMID: 655387.29. Lim BO, Seo TW, Shin HM, Park DK, Kim SU, Cho KH, Kim HC. Effect of betulae platyphyllae cortex on free radical in diabetic rats induced by streptozotocin. Korean J Herbol. 2000; 15:69–77.30. Torres-Piedra M, Ortiz-Andrade R, Villalobos-Molina R, Singh N, Medina-Franco JL, Webster SP, Binnie M, Navarrete-Vázquez G, Estrada-Soto S. A comparative study of flavonoid analogues on streptozotocin-nicotinamide induced diabetic rats: Quercetin as a potential antidiabetic agent acting via 11beta-hydroxysteroid dehydrogenase type 1 inhibition. Eur J Med Chem. 2010; 45:2606–2612. PMID: 20346546.31. Sharma B, Balomajumder C, Roy P. Hypoglycemic and hypolipidemic effects of flavonoid rich extract from Eugenia jambolana seeds on streptozotocin induced diabetic rats. Food Chem Toxicol. 2008; 46:2376–2383. PMID: 18474411.32. Lü H, Chen J, Li WL, Ren BR, Wu JL, Zhang HQ. Hypoglycemic effect of the total flavonoid fraction from Folium Eriobotryae. Phytomedicine. 2009; 16:967–971. PMID: 19427773.33. Kang SJ, Bao CL, Park S, Kim AJ. Hypoglycemic effect of Rehmannie radix preparata (Sookjihwang) extract in streptozotocin-induced diabetic rats. Nutr Res Pract. 2010; 4:438–442. PMID: 21103092.34. Singab AN, El-Beshbishy HA, Yonekawa M, Nomura T, Fukai T. Hypoglycemic effect of Egyptian Morus alba root bark extract: Effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J Ethnopharmacol. 2005; 14:333–338. PMID: 15885940.35. Chen F, Nakashima N, Kimura I, Kimura M. Hypoglycemic activity and mechanisms of extracts from mulberry leaves (Folium mori) and Cortex mori Radicis in streptozotocin-induced diabetic mice. Yakugaku Zasshi. 1995; 115:476–482. PMID: 7666358.36. Kim SG, Choi J, Park HJ, Lee SM, Jung HJ. Anti-hyperlipidemic effects of the flavonoid-rich fraction from the methanol extract of Orostachys japonicus in rats. Korean J Pharmacogn. 2009; 40:51–58.37. Felig P, Marliss E, Ohman JL, Cahill CF Jr. Plasma amino acid levels in diabetic ketoacidosis. Diabetes. 1970; 19:727–728. PMID: 4990780.
Article38. Singh SN, Vats P, Suri S, Shyam R, Kumria MM, Ranganathan S, Sridharan K. Effect of an antidiabetic extract of Catharanthus roseus on enzymic activities in streptozotocin induced diabetic rats. J Ethnopharmacol. 2001; 76:269–277. PMID: 11448549.39. Grover JK, Vats V, Yadav S. Effect of feeding aqueous extract of Pterocarpus marsupium on glycogen content of tissues and the key enzymes of carbohydrate metabolism. Mol Cell Biochem. 2002; 241:53–59. PMID: 12482025.40. Latha M, Pari L. Preventive effects of Cassia auriculata L. flowers on brain lipid peroxidation in rats treated with streptozotocin. Mol Cell Biochem. 2003; 243:23–28. PMID: 12619885.41. Venkateswaran S, Pari L. Antioxidant effect of Phaseolus vulgaris in streptozotocin-induced diabetic rats. Asia Pac J Clin Nutr. 2002; 11:206–209. PMID: 12230234.42. Wakame K. Protective effects of active hexose correlated compound (AHCC) on the onset of diabetes induced by streptozotocin in the rat. Biomed Res. 1999; 20:145–152.
Article43. Jang MJ, Rhee SJ. Antioxidant effects of mixture of mulberry leaves and silkworm powder on the plasma and liver in streptozotocin-induced diabetic rats. J Food Sci Nutr. 2004; 9:346–351.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypoglycemic effect of Rehmannie Radix Preparata (Sookjihwang) extract in streptozotocin-induced diabetic rats
- Proliferation of Cultured Vascular Smooth Muscle Cells(VSMCs) Obtained from Aortas of Insulin Dependent Diabetic Rats
- The antidiabetic effects of an herbal formula composed of Alnus hirsuta, Rosa davurica, Acanthopanax senticosus and Panax schinseng in the streptozotocin-induced diabetic rats
- Effects of Edible and Medicinal Plants Intake on Blood Glucose, Glycogen and Protein Levels in Streptozotocin Induced Diabetic Rats
- Effects of Polygonatum odoratum on In vivo Insulin Activity in Streptozotocin-Induced Diabetic Rats