Korean Asthma Guideline 2014: Summary of Major Updates to the Korean Asthma Guideline 2014
- Affiliations
-
- 1Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Pulmonary, Allergy and Critical Care Medicine, Hallym University Kangdong Sacred Heart Hospital, Seoul, Korea.
- 3Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Division of Pulmonary, Allergy and Critical Care Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea.
- 5Department of Pulmonary and Critical Care Medicine, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Korea. khyou@kuh.ac.kr kwan-kim@catholic.ac.kr
- 7Department of Internal Medicine, Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu, Korea. khyou@kuh.ac.kr kwan-kim@catholic.ac.kr
- KMID: 2326678
- DOI: http://doi.org/10.4046/trd.2016.79.3.111
Abstract
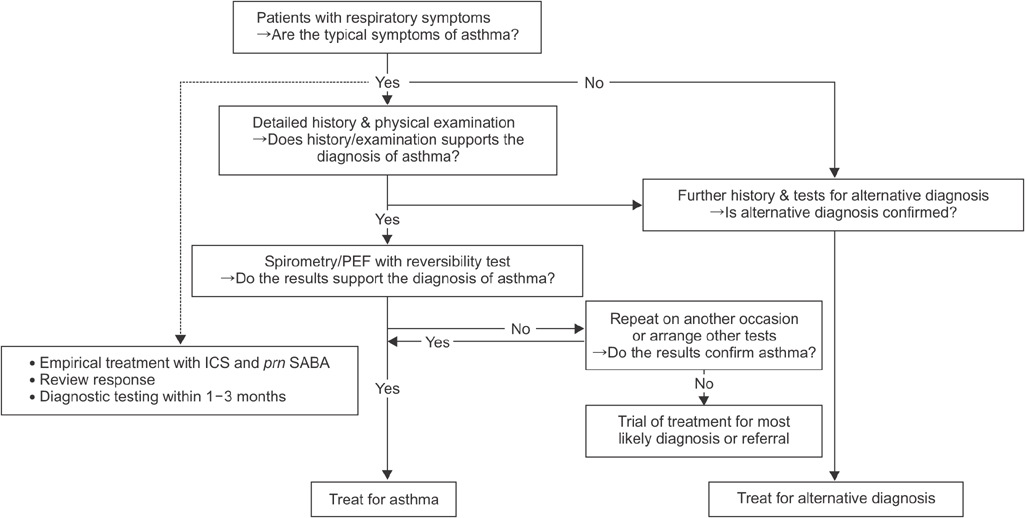
- Asthma is a prevalent and serious health problem in Korea. Recently, the Korean Asthma Guideline has been updated by The Korean Academy of Tuberculosis and Respiratory Diseases (KATRD) in an effort to improve the clinical management of asthma. This guideline focuses on adult patients with asthma and aims to deliver up to date scientific evidence and recommendations to general physicians for the management of asthma. For this purpose, this guideline was updated following systematic review and meta-analysis of recent studies and adapting some points of international guidelines (Global Initiative for Asthma [GINA] report 2014, National Asthma Education and Prevention Program [NAEPP] 2007, British Thoracic Society [BTS/SIGN] asthma guideline 2012, and Canadian asthma guideline 2012). Updated issues include recommendations derived using the population, intervention, comparison, and outcomes (PICO) model, which produced 20 clinical questions on the management of asthma. It also covers a new definition of asthma, the importance of confirming various airflow limitations with spirometry, the epidemiology and the diagnostic flow of asthma in Korea, the importance and evidence for inhaled corticosteroids (ICS) and ICS/formoterol as a single maintenance and acute therapy in the stepwise management of asthma, assessment of severity of asthma and management of exacerbation, and an action plan to cope with exacerbation. This guideline includes clinical assessments, and treatment of asthma-chronic obstructive pulmonary disease overlap syndrome, management of asthma in specific conditions including severe asthma, elderly asthma, cough variant asthma, exercise-induced bronchial contraction, etc. The revised Korean Asthma Guideline is expected to be a useful resource in the management of asthma.
MeSH Terms
Figure
Cited by 8 articles
-
Increased Risk of Exacerbation in Asthma Predominant Asthma–Chronic Obstructive Pulmonary Disease Overlap Syndrome
Jisoo Park, Eun-Kyung Kim, Mi-Ae Kim, Tae-Hyung Kim, Jung Hyun Chang, Yon Ju Ryu, Sei Won Lee, Yeon-Mok Oh, Suk Joong Yong, Won-Il Choi, Kwang Ha Yoo, Ji-Hyun Lee
Tuberc Respir Dis. 2018;81(4):289-298. doi: 10.4046/trd.2017.0064.Dilemma of Asthma Treatment in Mild Patients
You Sook Cho, Yeon-Mok Oh
Tuberc Respir Dis. 2019;82(3):190-193. doi: 10.4046/trd.2018.0013.Lung Function Trajectory Types in Never-Smoking Adults With Asthma: Clinical Features and Inflammatory Patterns
Joo-Hee Kim, Hun Soo Chang, Seung Woo Shin, Dong Gyu Baek, Ji-Hye Son, Choon-Sik Park, Jong-Sook Park
Allergy Asthma Immunol Res. 2018;10(6):614-627. doi: 10.4168/aair.2018.10.6.614.Efficacy and Safety of Benralizumab for Korean Patients With Severe, Uncontrolled Eosinophilic Asthma
Hae-Sim Park, Sang Haak Lee, Sook Young Lee, Mi-Kyeong Kim, Byung Jae Lee, Viktoria Werkström, Peter Barker, James G. Zangrilli
Allergy Asthma Immunol Res. 2019;11(4):508-518. doi: 10.4168/aair.2019.11.4.508.Increasing Prevalence and Mortality of Asthma With Age in Korea, 2002–2015: A Nationwide, Population-Based Study
Eunyoung Lee, Anhye Kim, Young-Min Ye, Sang-Eun Choi, Hae-Sim Park
Allergy Asthma Immunol Res. 2020;12(3):467-484. doi: 10.4168/aair.2020.12.3.467.Prevalence of Self-reported Allergic Diseases and IgE Levels: A 2010 KNHANES Analysis
Hye Jung Park, Eun-Jin Kim, Dankyu Yoon, Jeom Kyu Lee, Woo-Sung Chang, Yoen-Mi Lim, Jung-Won Park, Joo-Shil Lee
Allergy Asthma Immunol Res. 2017;9(4):329-339. doi: 10.4168/aair.2017.9.4.329.Effects of Education about Action Plans according to Self-Monitoring on Self-Management Adherence, Knowledge, Symptom Control, and Quality of Life among Adult Asthma Patients: A Randomized Controlled Trial
Ja Yun Choi, Young-Ran Kweon
J Korean Acad Nurs. 2017;47(5):613-623. doi: 10.4040/jkan.2017.47.5.613.Inverse Relationship between Adenoid Size and Asthma or Atopy in Children: A Preliminary Study
Yangseop Noh, Ji-Eun Choi, Kyung Eun Lee, Seung-Kyu Chung, Sang Duk Hong, Hyo Yeol Kim
Korean J Otorhinolaryngol-Head Neck Surg. 2020;63(9):409-414. doi: 10.3342/kjorl-hns.2019.00913.
Reference
-
1. World Health Organization. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach [Internet]. Geneva: World Health Organization;2007. cited 2016 Jan 3. Available from: http://www.who.int/gard/publications/GARD_Manual/en/.2. Park HS, Choi GS, Cho JS, Kim YY. Epidemiology and current status of allergic rhinitis, asthma, and associated allergic diseases in Korea: ARIA Asia-Pacific workshop report. Asian Pac J Allergy Immunol. 2009; 27:167–171.3. The clinical guideline of bronchial asthma [Internet]. Seoul: The Korean Academy of Tuberculosis and Respiratory Diseases;2005. cited 2016 Jan 3. Available from: http://www.lungkorea.org/thesis/guide.php.4. The Korean Academy of Tuberculosis and Respiratory Diseases. The Korean asthma guideline for adults: revised in 2014. Seoul: The Korean Academy of Tuberculosis and Respiratory Diseases;2014.5. Global Initiative for Asthma. Global strategy for asthma management and prevention. NHLBI/WHO Workshop report. Bethesda: National Heart, Lung and Blood Institute, National Institutes of Health;2014.6. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007; 120:5 Suppl. S94–138.7. British Thoracic Society Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax. 2008; 63:Suppl 4. iv1–iv121.8. Lougheed MD, Leniere C, Ducharme FM, Licskai C, Dell SD, Rowe BH, et al. Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults: executive summary. Can Respir J. 2012; 19:e81–e88.9. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development,reporting and evaluation in health care. CMAJ. 2010; 182:E839–E842.10. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004; 328:1490.11. Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011; 64:380–382.12. Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) Program.GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004; 59:469–478.13. Cho SH, Park HW, Rosenberg DM. The current status of asthma in Korea. J Korean Med Sci. 2006; 21:181–187.14. Lee YH, Yoon SJ, Kim EJ, Kim YA, Seo HY, Oh IH. Economic burden of asthma in Korea. Allergy Asthma Proc. 2011; 32:35–40.15. Statics Korea. Annual report on the cause of death statistics. Daejeon: Statics Korea;2013.16. Jeffery PK, Godfrey RW, Adelroth E, Nelson F, Rogers A, Johansson SA. Effects of treatment on airway inflammation and thickening of basement membrane reticular collagen in asthma: a quantitative light and electron microscopic study. Am Rev Respir Dis. 1992; 145(4 Pt 1):890–899.17. Juniper EF, Kline PA, Vanzieleghem MA, Ramsdale EH, O'Byrne PM, Hargreave FE. Effect of long-term treatment with an inhaled corticosteroid (budesonide) on airway hyperresponsiveness and clinical asthma in nonsteroid-dependent asthmatics. Am Rev Respir Dis. 1990; 142:832–836.18. Pauwels RA, Lofdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma: formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997; 337:1405–1411.19. Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000; 343:332–336.20. Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database Syst Rev. 2010; (5):CD005535.21. Cates CJ, Karner C. Combination formoterol and budesonide as maintenance and reliever therapy versus current best practice (including inhaled steroid maintenance), for chronic asthma in adults and children. Cochrane Database Syst Rev. 2013; (4):CD007313.22. Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009; 180:59–99.23. Manser R, Reid D, Abramson M. Corticosteroids for acute severe asthma in hospitalised patients. Cochrane Database Syst Rev. 2001; (1):CD001740.24. Rowe BH, Bota GW, Fabris L, Therrien SA, Milner RA, Jacono J. Inhaled budesonide in addition to oral corticosteroids to prevent asthma relapse following discharge from the emergency department: a randomized controlled trial. JAMA. 1999; 281:2119–2126.25. Kerstjens HA, Disse B, Schroder-Babo W, Bantje TA, Gahlemann M, Sigmund R, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. J Allergy Clin Immunol. 2011; 128:308–314.26. Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012; 367:1198–1207.27. Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2014; (1):CD003137.28. Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double-blind placebo-controlled trial. Lancet. 2001; 357:2007–2011.29. D'Amato G, Stanziola A, Sanduzzi A, Liccardi G, Salzillo A, Vitale C, et al. Treating severe allergic asthma with anti-IgE monoclonal antibody (omalizumab): a review. Multidiscip Respir Med. 2014; 9:23.30. Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014; (1):CD003559.31. Castro M, Rubin AS, Laviolette M, Fiterman J, De Andrade Lima M, Shah PL, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010; 181:116–124.32. Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, Olivenstein R, et al. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med. 2011; 11:8.33. Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013; 68:322–329.34. Cameron EJ, Chaudhuri R, Mair F, McSharry C, Greenlaw N, Weir CJ, et al. Randomised controlled trial of azithromycin in smokers with asthma. Eur Respir J. 2013; 42:1412–1415.35. Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, et al. An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 2013; 187:1016–1027.36. Gluck JC, Gluck PA. The effect of pregnancy on the course of asthma. Immunol Allergy Clin North Am. 2006; 26:63–80.