World J Mens Health.
2014 Aug;32(2):76-82. 10.5534/wjmh.2014.32.2.76.
A Milk Protein, Casein, as a Proliferation Promoting Factor in Prostate Cancer Cells
- Affiliations
-
- 1Department of Urology, Pusan National University Yangsan Hospital, Yangsan, Korea. lsd@pusan.ac.kr
- 2Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Korea.
- 3Institute for Medical Sciences, Ajou University School of Medicine, Suwon, Korea.
- 4Genitourinary Cancer Branch, National Cancer Center, Goyang, Korea.
- KMID: 2320796
- DOI: http://doi.org/10.5534/wjmh.2014.32.2.76
Abstract
- PURPOSE
Despite most epidemiologic studies reporting that an increase in milk intake affects the growth of prostate cancer, the results of experimental studies are not consistent. In this study, we investigated the proliferation of prostate cancer cells treated with casein, the main protein in milk.
MATERIALS AND METHODS
Prostate cancer cells (LNCaP and PC3), lung cancer cells (A459), stomach cancer cells (SNU484), breast cancer cells (MCF7), immortalized human embryonic kidney cells (HEK293), and immortalized normal prostate cells (RWPE1) were treated with either 0.1 or 1 mg/mL of alpha-casein and total casein extracted from bovine milk. Treatments were carried out in serum-free media for 72 hours. The proliferation of each cell line was evaluated by an 3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay.
RESULTS
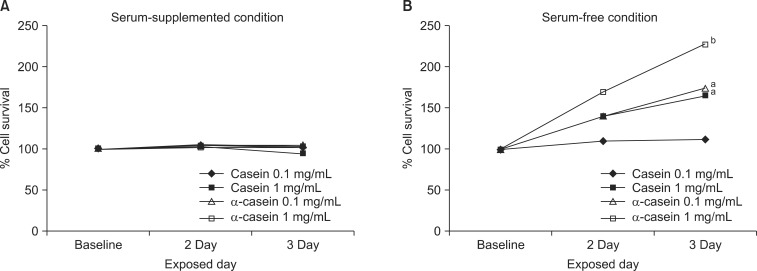
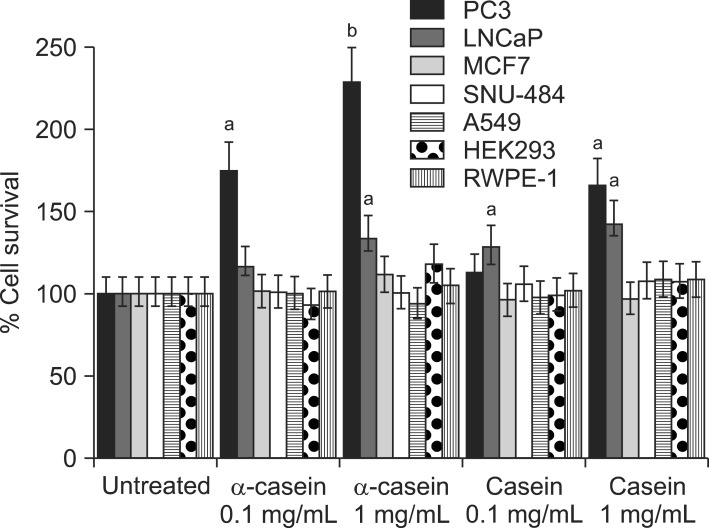
alpha-Casein and total casein did not affect the proliferations of RWPE1, HEK293, A459, SNU484, MCF7, HEK293, or RWPE1 cells. However, PC3 cells treated with 1 mg/mL of alpha-casein and casein showed increased proliferation (228% and 166%, respectively), and the proliferation of LNCaP cells was also enhanced by 134% and 142%, respectively. The proliferation mechanism of alpha-casein in PC3 and LNCaP cells did not appear to be related to the induction of Insulin-like growth factor-1 (IGF-1), since the level of IGF-1 did not change upon the supplementation of casein.
CONCLUSIONS
The milk protein, casein, promotes the proliferation of prostate cancer cells such as PC3 and LNCaP.
Keyword
MeSH Terms
Figure
Reference
-
1. Chan JM, Stampfer MJ, Ma J, Gann PH, Gaziano JM, Giovannucci EL. Dairy products, calcium, and prostate cancer risk in the Physicians' Health Study. Am J Clin Nutr. 2001; 74:549–554. PMID: 11566656.
Article2. Rohrmann S, Platz EA, Kavanaugh CJ, Thuita L, Hoffman SC, Helzlsouer KJ. Meat and dairy consumption and subsequent risk of prostate cancer in a US cohort study. Cancer Causes Control. 2007; 18:41–50. PMID: 17315319.
Article3. Raimondi S, Mabrouk JB, Shatenstein B, Maisonneuve P, Ghadirian P. Diet and prostate cancer risk with specific focus on dairy products and dietary calcium: a case-control study. Prostate. 2010; 70:1054–1065. PMID: 20232354.
Article4. Kurahashi N, Inoue M, Iwasaki M, Sasazuki S, Tsugane AS. Japan Public Health Center-Based Prospective Study Group. Dairy product, saturated fatty acid, and calcium intake and prostate cancer in a prospective cohort of Japanese men. Cancer Epidemiol Biomarkers Prev. 2008; 17:930–937. PMID: 18398033.
Article5. Tseng M, Breslow RA, Graubard BI, Ziegler RG. Dairy, calcium, and vitamin D intakes and prostate cancer risk in the National Health and Nutrition Examination Epidemiologic follow-up study cohort. Am J Clin Nutr. 2005; 81:1147–1154. PMID: 15883441.
Article6. Gao X, LaValley MP, Tucker KL. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst. 2005; 97:1768–1777. PMID: 16333032.
Article7. Huncharek M, Muscat J, Kupelnick B. Dairy products, dietary calcium and vitamin D intake as risk factors for prostate cancer: a meta-analysis of 26,769 cases from 45 observational studies. Nutr Cancer. 2008; 60:421–441. PMID: 18584476.
Article8. Park SY, Murphy SP, Wilkens LR, Stram DO, Henderson BE, Kolonel LN. Calcium, vitamin D, and dairy product intake and prostate cancer risk: the multiethnic cohort study. Am J Epidemiol. 2007; 166:1259–1269. PMID: 17925283.
Article9. Rodriguez C, McCullough ML, Mondul AM, Jacobs EJ, Fakhrabadi-Shokoohi D, Giovannucci EL, et al. Calcium, dairy products, and risk of prostate cancer in a prospective cohort of United States men. Cancer Epidemiol Biomarkers Prev. 2003; 12:597–603. PMID: 12869397.10. Qin LQ, Wang PY, Kaneko T, Hoshi K, Sato A. Estrogen: one of the risk factors in milk for prostate cancer. Med Hypotheses. 2004; 62:133–142. PMID: 14729019.
Article11. Saikali Z, Setya H, Singh G, Persad S. Role of IGF-1/IGF-1R in regulation of invasion in DU145 prostate cancer cells. Cancer Cell Int. 2008; 8:10. PMID: 18598360.
Article12. Parodi PW. A role for milk proteins and their peptides in cancer prevention. Curr Pharm Des. 2007; 13:813–828. PMID: 17430183.
Article13. van Boekel MA, Weerens CN, Holstra A, Scheidtweiler CE, Alink GM. Antimutagenic effects of casein and its digestion products. Food Chem Toxicol. 1993; 31:731–737. PMID: 8225131.
Article14. Jongen WM, van Boekel MA, van Broekhoven LW. Inhibitory effect of cheese and some food constituents on mutagenicity generated in Vicia faba after treatment with nitrite. Food Chem Toxicol. 1987; 25:141–145. PMID: 3557236.
Article15. Phelan M, Aisling Aherne S, O'Sullivan D, FitzGerald RJ, O'Brien NM. Growth inhibitory effects of casein hydrolysates on human cancer cell lines. J Dairy Res. 2010; 77:176–182. PMID: 20053315.
Article16. Tate PL, Bibb R, Larcom LL. Milk stimulates growth of prostate cancer cells in culture. Nutr Cancer. 2011; 63:1361–1366. PMID: 22043817.
Article17. Nielsen TS, Höjer A, Gustavsson AM, Hansen-Møller J, Purup S. Proliferative effect of whey from cows' milk varying in phyto-oestrogens in human breast and prostate cancer cells. J Dairy Res. 2012; 79:143–149. PMID: 22280936.
Article18. Jenness R. Comparative aspects of milk proteins. J Dairy Res. 1979; 46:197–210. PMID: 469043.
Article19. Swaisgood HE. Review and update of casein chemistry. J Dairy Sci. 1993; 76:3054–3061. PMID: 8227630.
Article20. McIntosh GH, Regester GO, Le Leu RK, Royle PJ, Smithers GW. Dairy proteins protect against dimethylhydrazine-induced intestinal cancers in rats. J Nutr. 1995; 125:809–816. PMID: 7722681.21. Papenburg R, Bounous G, Fleiszer D, Gold P. Dietary milk proteins inhibit the development of dimethylhydrazine-induced malignancy. Tumour Biol. 1990; 11:129–136. PMID: 2343238.
Article22. Bonuccelli G, Castello-Cros R, Capozza F, Martinez-Outschoorn UE, Lin Z, Tsirigos A, et al. The milk protein α-casein functions as a tumor suppressor via activation of STAT1 signaling, effectively preventing breast cancer tumor growth and metastasis. Cell Cycle. 2012; 11:3972–3982. PMID: 23047602.
Article23. Pettersson A, Kasperzyk JL, Kenfield SA, Richman EL, Chan JM, Willett WC, et al. Milk and dairy consumption among men with prostate cancer and risk of metastases and prostate cancer death. Cancer Epidemiol Biomarkers Prev. 2012; 21:428–436. PMID: 22315365.
Article24. Kolb AF, Huber RC, Lillico SG, Carlisle A, Robinson CJ, Neil C, et al. Milk lacking α-casein leads to permanent reduction in body size in mice. PLoS One. 2011; 6:e21775. PMID: 21789179.
Article25. Chen KC, Peng CC, Peng RY, Su CH, Chiang HS, Yan JH, et al. Unique formosan mushroom Antrodia camphorata differentially inhibits androgen-responsive LNCaP and -independent PC-3 prostate cancer cells. Nutr Cancer. 2007; 57:111–121. PMID: 17516868.26. Dozmorov MG, Hurst RE, Culkin DJ, Kropp BP, Frank MB, Osban J, et al. Unique patterns of molecular profiling between human prostate cancer LNCaP and PC-3 cells. Prostate. 2009; 69:1077–1090. PMID: 19343732.
Article27. Corpet DE, Chatelin-Pirot V. Cooked casein promotes colon cancer in rats, may be because of mucosal abrasion. Cancer Lett. 1997; 114:89–90. PMID: 9103260.
Article28. Phelan M, Aherne-Bruce A, O'Sullivan D, FitzGerald RJ, O'Brien NM. Potential bioactive effects of casein hydrolysates on human cultured cells. Int Dairy J. 2009; 19:279–285.
Article29. Perego S, Cosentino S, Fiorilli A, Tettamanti G, Ferraretto A. Casein phosphopeptides modulate proliferation and apoptosis in HT-29 cell line through their interaction with voltage-operated L-type calcium channels. J Nutr Biochem. 2012; 23:808–816. PMID: 21840696.
Article30. Kimura T, Murakawa Y, Ohno M, Ohtani S, Higaki K. Gastrointestinal absorption of recombinant human insulin-like growth factor-I in rats. J Pharmacol Exp Ther. 1997; 283:611–618. PMID: 9353376.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Urticaria Developed by Antianemics which Contain Cow's Milk Protein (casein) or Egg White Protein (ovalbumin)
- Cow's Milk Protein-specific IgE Concentrations in Two Age Groups of Children with cow's Milk Allergy
- Development and properties of hypoallergenic infant formula
- Effect of mastitis on raw milk compositional quality
- The Effect of A2 Milk on Gastrointestinal Symptoms in Comparison to A1/A2 Milk: A Single-center, Randomized, Double-blind, Cross-over Study