Tuberc Respir Dis.
2015 Apr;78(2):128-132. 10.4046/trd.2015.78.2.128.
Two Cases of Bronchopulmonary Dysplasia of Similar Appearance in Adult Monozygotic Twin: Pathology and Computed Tomographic Findings
- Affiliations
-
- 1Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea. cem@ewha.ac.kr
- 2Department of Radiology, Ewha Womans University School of Medicine, Seoul, Korea.
- 3Department of Pathology, Ewha Womans University School of Medicine, Seoul, Korea.
- KMID: 2320606
- DOI: http://doi.org/10.4046/trd.2015.78.2.128
Abstract
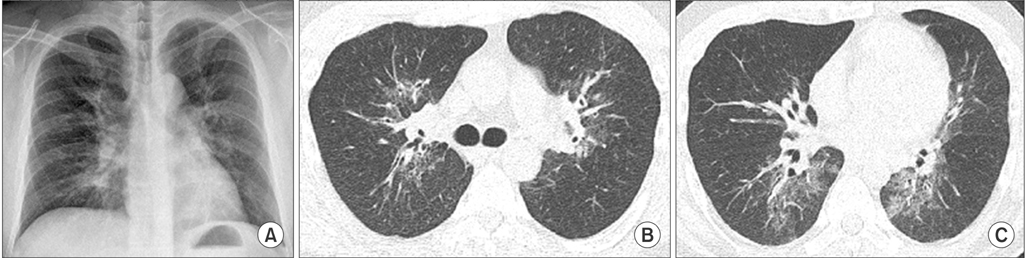
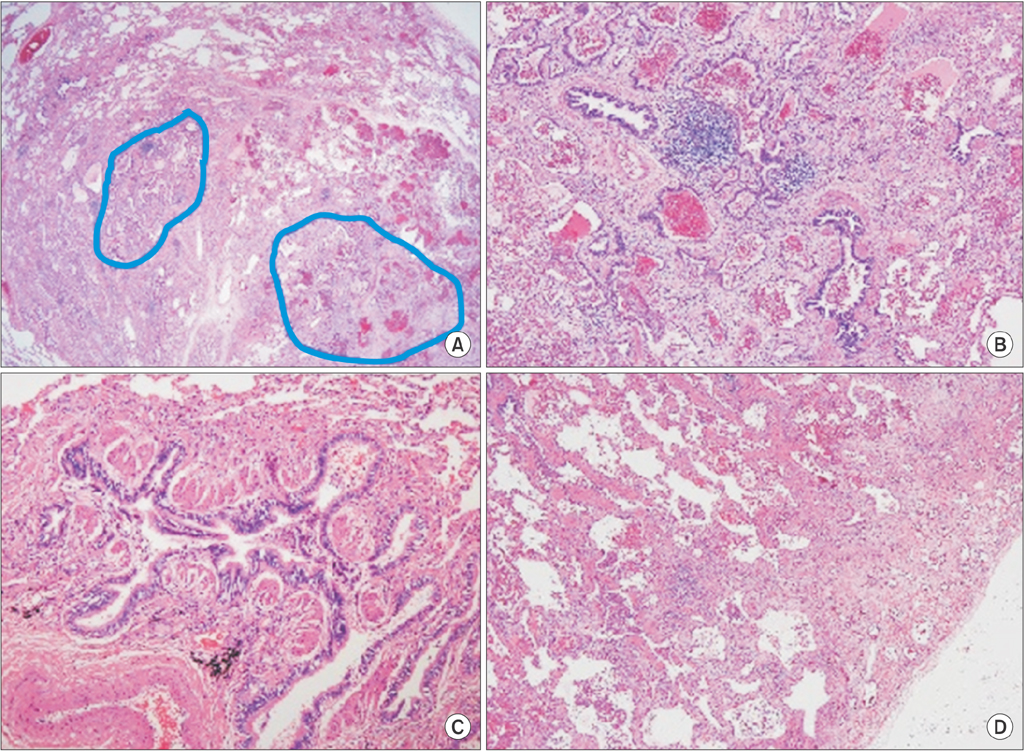
- Bronchopulmonary dysplasia (BPD) is related to decreased lung function throughout life. However, the pathology and radiology pattern of BPD of adults are not documented well yet. In this case report, we present BPD case of an adult monozygotic twin showing nearly identical lesions on chest computed tomography (CT). CT images showed mixed areas of ground-glass and reticular opacities in both lungs. They had common histories of pneumonias requiring mechanical ventilations in period of infants. Pulmonary function test of one patient showed a pulmonary insufficiency with airway obstruction. Pathologic findings showed bronchiolar hyperplasia and peribronchiolar fibrosis which was similar to classic BPD patients. Our twin case report might help provide distinguishing pathology and radiology pattern of an adult pulmonary sequelaes of BPD. It might be reasonable to make close follow-up for BPD patients to evaluate the long-term outcomes of BPD survivors.
MeSH Terms
Figure
Reference
-
1. Walsh MC, Szefler S, Davis J, Allen M, Van Marter L, Abman S, et al. Summary proceedings from the bronchopulmonary dysplasia group. Pediatrics. 2006; 117(3 Pt 2):S52–S56.2. Bhandari A, McGrath-Morrow S. Long-term pulmonary outcomes of patients with bronchopulmonary dysplasia. Semin Perinatol. 2013; 37:132–137.3. Howling SJ, Northway WH Jr, Hansell DM, Moss RB, Ward S, Muller NL. Pulmonary sequelae of bronchopulmonary dysplasia survivors: high-resolution CT findings. AJR Am J Roentgenol. 2000; 174:1323–1326.4. Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, et al. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J. 2008; 32:321–328.5. Bancalari E. Changes in the pathogenesis and prevention of chronic lung disease of prematurity. Am J Perinatol. 2001; 18:1–9.6. Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006; 117:1901–1906.7. Lavoie PM, Pham C, Jang KL. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics. 2008; 122:479–485.8. Bhandari A, Bhandari V. "New" bronchopulmonary dysplasia: a clinical review. Clin Pulm Med. 2011; 18:137–143.9. Vrijlandt EJ, Gerritsen J, Boezen HM, Grevink RG, Duiverman EJ. Lung function and exercise capacity in young adults born prematurely. Am J Respir Crit Care Med. 2006; 173:890–896.10. Vrijlandt EJ, Boezen HM, Gerritsen J, Stremmelaar EF, Duiverman EJ. Respiratory health in prematurely born preschool children with and without bronchopulmonary dysplasia. J Pediatr. 2007; 150:256–261.11. Landry JS, Chan T, Lands L, Menzies D. Long-term impact of bronchopulmonary dysplasia on pulmonary function. Can Respir J. 2011; 18:265–270.12. Aukland SM, Halvorsen T, Fosse KR, Daltveit AK, Rosendahl K. High-resolution CT of the chest in children and young adults who were born prematurely: findings in a populationbased study. AJR Am J Roentgenol. 2006; 187:1012–1018.13. Bhandari A, Panitch HB. Pulmonary outcomes in bronchopulmonary dysplasia. Semin Perinatol. 2006; 30:219–226.14. Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, et al. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med. 2010; 182:237–245.15. Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol. 2006; 30:179–184.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Thanatophoric Dysplasia in Monozygotic Twin: Prenatal Diagnosis, Clinical, Radiological and Pathological Findings
- A case of monozygotic twin with Down syndrome
- 3 Cases of Monozygotic Twin Pregnancy after IVF-ET
- A Case of Brain Damage in Surviving Monozygotic Twin After Intrauterine Death of Co-Twin
- A cass of Twin to Twin Transfusion Syndrome