Tuberc Respir Dis.
2012 May;72(5):452-456.
Disseminated Mycobacterium intracellulare Infection in an Immunocompetent Host
- Affiliations
-
- 1Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. shimts@amc.seoul.kr
Abstract
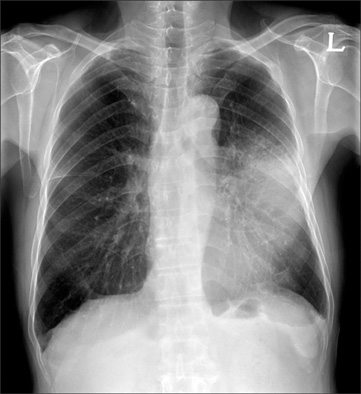
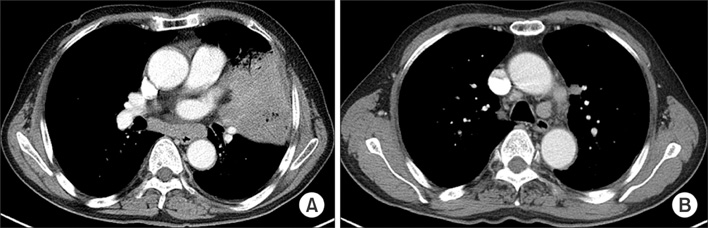
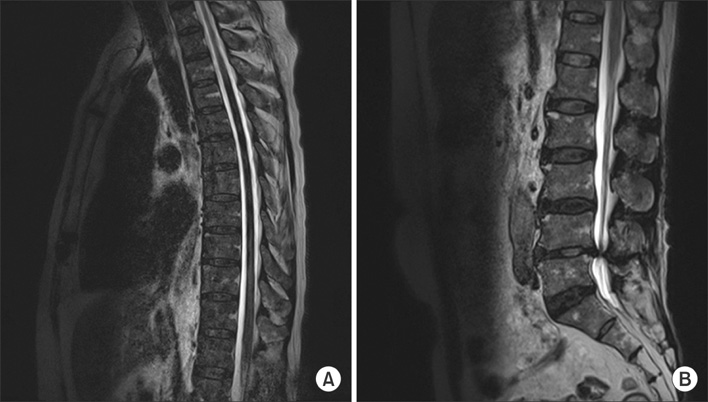
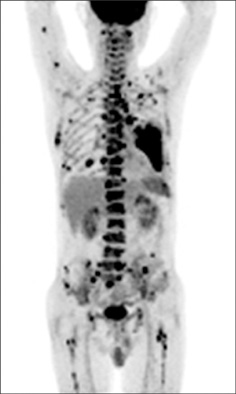
- Disseminated Mycobacterium avium complex (MAC) infection can occur in immunocompromised patients, and rarely in immunocompetent subjects. Due to the extensive distribution of the disease, clinical presentation of disseminated MAC may mimic malignancies, and thorough examinations are required in order to make accurate diagnosis. We report a case of disseminated Mycobacterium intracellulare disease in an immunocompetent patient, which involved the lung, lymph nodes, spleen, and multiple bones. F-18 fluorodeoxyglucose positron-emission tomography imaging showed multiple hypermetabolic lesions, which are suggestive of typical hematogenous metastasis. However, there was no evidence of malignancy in serial biopsies, and M. intracellulare was repeatedly cultured from respiratory specimens and bones. Herein, we should know that disseminated infection can occur in the immunocompetent subjects, and it can mimic malignancies.
Keyword
MeSH Terms
Figure
Reference
-
1. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007. 175:367–416.2. Song JY, Park CW, Kee SY, Choi WS, Kang EY, Sohn JW, et al. Disseminated Mycobacterium avium complex infection in an immunocompetent pregnant woman. BMC Infect Dis. 2006. 6:154.3. Kim SY, Oh DW, Yu JH, Kim D, Noh S, Roh J, et al. A case of disseminated Mycobacterium intracellulare infection in an immunocompromised host. Tuberc Respir Dis. 2009. 67:32–36.4. Chung JW, Cha YJ, Oh DJ, Nam WJ, Kim SH, Lee MK, et al. Disseminated Mycobacterium avium complex infection in a non-HIV-infected patient undergoing continuous ambulatory peritoneal dialysis. Korean J Lab Med. 2010. 30:166–170.5. Lowe VJ, Naunheim KS. Positron emission tomography in lung cancer. Ann Thorac Surg. 1998. 65:1821–1829.6. Meller J, Sahlmann CO, Scheel AK. 18F-FDG PET and PET/CT in fever of unknown origin. J Nucl Med. 2007. 48:35–45.7. Medical Section of the American Lung Association. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Am J Respir Crit Care Med. 1997. 156(2 Pt 2):S1–S25.8. Okada H, Yoshioka K. Acute disseminated encephalomyelitis associated with meningitis due to Mycobacterium intracellulare. Intern Med. 2010. 49:2113–2116.9. Azzam HC, Gahunia MK, Sae-Tia S, Santoro J. Mycobacterium avium-avium-associated typhlitis mimicking appendicitis in an immunocompetent host. Am J Med Sci. 2009. 337:218–220.10. Nightingale SD, Byrd LT, Southern PM, Jockusch JD, Cal SX, Wynne BA. Incidence of Mycobacterium avium-intracellulare complex bacteremia in human immunodeficiency virus-positive patients. J Infect Dis. 1992. 165:1082–1085.11. Kasperbauer SH, Daley CL. Diagnosis and treatment of infections due to Mycobacterium avium complex. Semin Respir Crit Care Med. 2008. 29:569–576.12. Chou CH, Chen HY, Chen CY, Huang CT, Lai CC, Hsueh PR. Clinical features and outcomes of disseminated infections caused by non-tuberculous mycobacteria in a university hospital in Taiwan, 2004-2008. Scand J Infect Dis. 2011. 43:8–14.13. Zhuang H, Pourdehnad M, Yamamoto AJ, Rossman MD, Alavi A. Intense F-18 fluorodeoxyglucose uptake caused by Mycobacterium avium intracellulare infection. Clin Nucl Med. 2001. 26:458.14. Bandoh S, Fujita J, Ueda Y, Tojo Y, Ishii T, Kubo A, et al. Uptake of fluorine-18-fluorodeoxyglucose in pulmonary Mycobacterium avium complex infection. Intern Med. 2003. 42:726–729.15. Sato M, Hiyama T, Kaito K, Hayashi Y, Okumura T. Usefulness of F-18 FDG PET/CT in the assessment of disseminated Mycobacterium avium complex infection. Ann Nucl Med. 2009. 23:757–762.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Disseminated Mycobacterium intracellulare Infection in an Immunocompromised Host
- Vertebral Osteomyelitis due to Mycobacterium intracellulare in an Immunocompetent Elderly Patient After Vertebroplasty
- A Case of Pulmonary and Endobronchial Mycobacterium avium Infection Presenting as an Acute Pneumonia in an Immunocompetent Patient
- A Case of Arthritis due to Mycobacterium Intracellulare in a Immunocompetent Patient
- Mycobacterium avium Infection Presenting as Endobronchial Lesions in an Immunocompetent Patient