Tuberc Respir Dis.
2011 Jul;71(1):30-36.
Usefulness of Vibration Response Imaging (VRI) for Pneumonia Patients
- Affiliations
-
- 1Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Korea. khyou@kuh.ac.kr
- 2Department of Internal Medicine, Konkuk University Chungju Hospital, Chungju, Korea.
Abstract
- BACKGROUND
Pneumonia is commonly seen in outpatient clinics. it is widely known as the most common cause of death from infectious disease. Pneumonia has been diagnosed by its typical symptoms, chest X-ray and blood tests. However, both chest X-rays and blood tests have limitations in diagnosis. Thus primary care clinicians usually have been constrained due to a lack of adequate diagnostic tools. Vibration response imaging (VRI) is a newly emerging diagnostic modality, and its procedure is non-invasive, radiation-free, and easy to handle. This study was designed to evaluate the diagnostic usefulness of the VRI test among pneumonia patients and to consider its correlation with other conventional tests such as Chest X-ray, laboratory tests and clinical symptoms.
METHODS
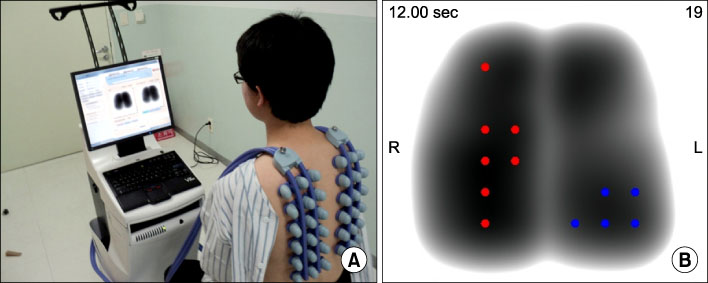
VRI was performed in 46 patients diagnosed with pneumonia in Konkuk University Medical Center. VRI was assessed in a private and quiet room twice: before and after the treatment. Sensors for VRI were placed on a patient's back at regular intervals; they detected pulmonary vibration energy produced when respiration occurred and presented as specific images. Any modifications either in chest X-ray, C-reactive protein (CRP), white blood cell count (WBC) or body temperature were compared with changes in VRI image during a given time course.
RESULTS
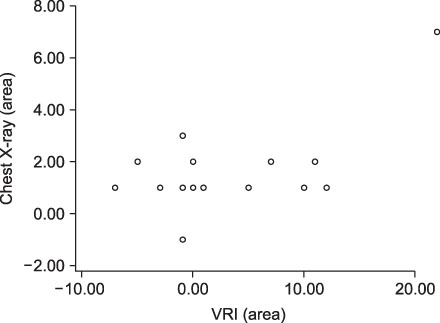
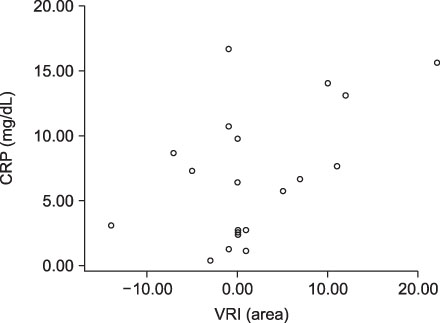
VRI, chest X-ray and CRP scores were significantly improved after treatment. Correlation between VRI and other tests was not clearly indicated among all patients. But relatively severe pneumonia patients showed correlations between VRI and chest X-ray, as well as between VRI and CRP.
CONCLUSION
This study demonstrates that VRI can be safely applied to patients with pneumonia.
Keyword
MeSH Terms
Figure
Reference
-
1. Niederman MS, Bass JB Jr, Campbell GD, Fein AM, Grossman RF, Mandell LA, et al. Guidelines for the initial management of adults with community-acquired pneumonia: diagnosis, assessment of severity, and initial antimicrobial therapy. American Thoracic Society. Medical Section of the American Lung Association. Am Rev Respir Dis. 1993. 148:1418–1426.2. Jokinen C, Heiskanen L, Juvonen H, Kallinen S, Karkola K, Korppi M, et al. Incidence of community-acquired pneumonia in the population of four municipalities in eastern Finland. Am J Epidemiol. 1993. 137:977–988.3. Almirall J, Bolíbar I, Vidal J, Sauca G, Coll P, Niklasson B, et al. Epidemiology of community-acquired pneumonia in adults: a population-based study. Eur Respir J. 2000. 15:757–763.4. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007. 44:Suppl 2. S27–S72.5. Metlay JP, Fine MJ. Testing strategies in the initial management of patients with community-acquired pneumonia. Ann Intern Med. 2003. 138:109–118.6. Flanders SA, Stein J, Shochat G, Sellers K, Holland M, Maselli J, et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am J Med. 2004. 116:529–535.7. Pasterkamp H, Kraman SS, Wodicka GR. Respiratory sounds. Advances beyond the stethoscope. Am J Respir Crit Care Med. 1997. 156:974–987.8. Loudon RG. The lung exam. Clin Chest Med. 1987. 8:265–272.9. Murphy RL, Vyshedskiy A, Power-Charnitsky VA, Bana DS, Marinelli PM, Wong-Tse A, et al. Automated lung sound analysis in patients with pneumonia. Respir Care. 2004. 49:1490–1497.10. Kompis M, Pasterkamp H, Wodicka GR. Acoustic imaging of the human chest. Chest. 2001. 120:1309–1321.11. Mor R, Kushnir I, Meyer JJ, Ekstein J, Ben-Dov I. Breath sound distribution images of patients with pneumonia and pleural effusion. Respir Care. 2007. 52:1753–1760.12. Maher TM, Gat M, Allen D, Devaraj A, Wells AU, Geddes DM. Reproducibility of dynamically represented acoustic lung images from healthy individuals. Thorax. 2008. 63:542–548.13. Dellinger RP, Parrillo JE, Kushnir A, Rossi M, Kushnir I. Dynamic visualization of lung sounds with a vibration response device: a case series. Respiration. 2008. 75:60–72.14. Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003. 58:377–382.15. Almirall J, Bolíbar I, Toran P, Pera G, Boquet X, Balanzó X, et al. Contribution of C-reactive protein to the diagnosis and assessment of severity of community-acquired pneumonia. Chest. 2004. 125:1335–1342.16. Castro-Guardiola A, Armengou-Arxé A, Viejo-Rodríguez A, Peñarroja-Matutano G, Garcia-Bragado F. Differential diagnosis between community-acquired pneumonia and non-pneumonia diseases of the chest in the emergency ward. Eur J Intern Med. 2000. 11:334–339.17. Hansson LO, Hedlund JU, Ortqvist AB. Sequential changes of inflammatory and nutritional markers in patients with community-acquired pneumonia. Scand J Clin Lab Invest. 1997. 57:111–118.18. Póvoa P, Coelho L, Almeida E, Fernandes A, Mealha R, Moreira P, et al. C-reactive protein as a marker of ventilator-associated pneumonia resolution: a pilot study. Eur Respir J. 2005. 25:804–812.19. Danesh J, Muir J, Wong YK, Ward M, Gallimore JR, Pepys MB. Risk factors for coronary heart disease and acute-phase proteins. A population-based study. Eur Heart J. 1999. 20:954–959.20. Hutchinson WL, Koenig W, Fröhlich M, Sund M, Lowe GD, Pepys MB. Immunoradiometric assay of circulating C-reactive protein: age-related values in the adult general population. Clin Chem. 2000. 46:934–938.21. Li J, Cai BQ. Characteristics of vibration response imaging in chronic obstructive pulmonary disease. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2009. 31:335–338.22. Elphick HE, Lancaster GA, Solis A, Majumdar A, Gupta R, Smyth RL. Validity and reliability of acoustic analysis of respiratory sounds in infants. Arch Dis Child. 2004. 89:1059–1063.23. Charleston-Villalobos S, Cortés-Rubiano S, González-Camarena R, Chi-Lem G, Aljama-Corrales T. Respiratory acoustic thoracic imaging (RATHI): assessing deterministic interpolation techniques. Med Biol Eng Comput. 2004. 42:618–626.24. Aagaard E, Maselli J, Gonzales R. Physician practice patterns: chest x-ray ordering for the evaluation of acute cough illness in adults. Med Decis Making. 2006. 26:599–605.25. Lynch T, Platt R, Gouin S, Larson C, Patenaude Y. Can we predict which children with clinically suspected pneumonia will have the presence of focal infiltrates on chest radiographs? Pediatrics. 2004. 113:e186–e189.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of vibration perception time in assessment of vibration sensory impairment
- Erection and ejaculation response to penile stimulation by vibration in patients with erectile dysfunction
- Partial Nucleotide Sequence of Porcine Parvovirus (VRI-1 Strain): Identification of the Putative Defective Genomes
- Prevalence of the Vibration Syndrome among Rock-drilers in the Anthracite Mining Area
- Apical Turn Vibratory Response Changes Following Cisplatin Application in the Living Guinea Pig Cochlea