Tuberc Respir Dis.
2011 Jul;71(1):15-23.
Comparison of Therapeutic Efficacy of Gefitinib and Erlotinib in Patients with Squamous Cell Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea. droij@chonnam.ac.kr
- 2Department of Internal Medicine, Seonam University College of Medicine, Gwangju, Korea.
Abstract
- BACKGROUND
Gefitinib and erlotinib are useful, molecular targeted agents in patients with non-small-cell lung cancer (NSCLC) who failed previous chemotherapy. We compared the efficacy and toxicity of two drugs in patients with squamous cell lung cancer, most of whom are male smokers.
METHODS
We retrospectively reviewed the clinical information on patients with NSCLC who were treated with gefitinib or erlotinib treatment at Chonnam National University Hwasun Hospital between July 2002 and November 2009. The overall response rate (ORR), overall survival (OS) and progression-free survival (PFS) were compared between the two drugs.
RESULTS
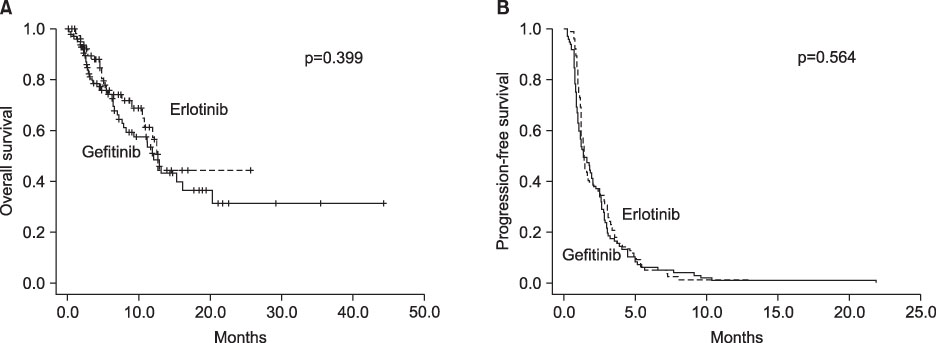
A total of 182 (100 gefitinib vs. 82 erlotinib) of 584 patients treated by targeted agents had squamous histology. Of the 182 patients, 167 (91.7%) were male and 159 (87.4%) were smokers. The ORR and disease control rate (DCR) were 4.9% and 40.6%, and there was no significant difference between gefitinib and erlotinib (ORR, 5.0% vs 4.8%; p=0.970; DCR, 40.0% vs 41.4%; p=0.439). The median OS in the gefitinib group was 12.1 months, and that in the erlotinib was 12.7 months (hazard ratio [HR], 1.282; 95% confidence interval [CI], 0.771~2.134; p=0.339). The median PFS for the gefitinib group was 1.40 months, compared with 1.37 months for the erlotinib group (HR, 1.092; 95% CI, 0.809~1.474; p=0.564). Skin rash > or =grade 3 was more common in erlotinib (12.2%) than gefitinib (1.0%, p=0.003) groups.
CONCLUSION
This retrospective study showed that the two drugs appear to have similar antitumor efficacy and toxicity except for skin rash.
MeSH Terms
Figure
Reference
-
1. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005. 353:123–132.2. Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet. 2005. 366:1527–1537.3. National Comprehensive Cancer Network: your best resource in the fight against cancer [Homepage]. National Comprehensive Cancer Network (NCCN). c2011. cited 2011 July 20. Fort Washington, PA: NCCN;Available from: http://www.nccn.org/index.asp.4. Cataldo VD, Gibbons DL, Pérez-Soler R, Quintás-Cardama A. Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med. 2011. 364:947–955.5. Emery IF, Battelli C, Auclair PL, Carrier K, Hayes DM. Response to gefitinib and erlotinib in non-small cell lung cancer: a restrospective study. BMC Cancer. 2009. 9:333.6. Kim ST, Lee J, Kim JH, Won YW, Sun JM, Yun J, et al. Comparison of gefitinib versus erlotinib in patients with nonsmall cell lung cancer who failed previous chemotherapy. Cancer. 2010. 116:3025–3033.7. Hong J, Kyung SY, Lee SP, Park JW, Jung SH, Lee JI, et al. Pemetrexed versus gefitinib versus erlotinib in previously treated patients with non-small cell lung cancer. Korean J Intern Med. 2010. 25:294–300.8. Lee JH, Lee KE, Ryu YJ, Chun EM, Chang JH. Comparison of gefitinib and erlotinib for patients with advanced non-small-cell lung cancer. Tuberc Respir Dis. 2009. 66:280–287.9. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009. 45:228–247.10. Cancer therapy evaluation program: common terminology criteria for adverse events (CTCAE) version 3.0. National Cancer Institute (NCI). 2006. cited 2011 July 20. Bethesda, MD: NCI;Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ docs/ctcaev3.pdf.11. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009. 361:947–957.12. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010. 362:2380–2388.13. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010. 11:121–128.14. Zhou C, Wung YI, Chen G, Feng J, Liu X, Wang C, et al. Efficacy results from the randomised phase III OPTIMAL (CTONG 0802) study comparing first-line erlotinib versus carboplatin plus gemcitabine, in chinese advanced non-small-cell lung cancer patients with EGFR activating mutations. In : Presented at European Society of Medical Oncology (ESMO) Congress; 2010 Oct 8-12; Milan, Italy.15. Massuti B, Morán T, Porta R, Queralt C, Cardenal F, Mayo C, et al. Multicenter prospective trial of customized erlotinib for advanced non-small cell lung cancer (NSCLC) patients (p) with epidermal growth factor receptor (EGFR) mutations: final results of the Spanish Lung Cancer Group (SLCG) trial. J Clin Oncol. 2009. 27:Suppl 15. 8023.16. Forbes SA, Bhamra G, Bamford S, Dawson E, Kok C, Clements J, et al. The catalogue of somatic mutations in cancer (COSMIC). Curr Protoc Hum Genet. 2008. Chapter 10:Unit 10.11.17. Miyamae Y, Shimizu K, Hirato J, Araki T, Tanaka K, Ogawa H, et al. Significance of epidermal growth factor receptor gene mutations in squamous cell lung carcinoma. Oncol Rep. 2011. 25:921–928.18. Park SH, Ha SY, Lee JI, Lee H, Sim H, Kim YS, et al. Epidermal growth factor receptor mutations and the clinical outcome in male smokers with wquamous cell carcinoma of lung. J Korean Med Sci. 2009. 24:448–452.19. Shukuya T, Takahashi T, Kaira R, Ono A, Nakamura Y, Tsuya A, et al. Efficacy of gefitinib for non-adenocarcinoma non-small-cell lung cancer patients harboring epidermal growth factor receptor mutations: a pooled analysis of published reports. Cancer Sci. 2011. 102:1032–1037.20. Baselga J, Rischin D, Ranson M, Calvert H, Raymond E, Kieback DG, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol. 2002. 20:4292–4302.21. Hidalgo M, Siu LL, Nemunaitis J, Rizzo J, Hammond LA, Takimoto C, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001. 19:3267–3279.22. Li J, Zhao M, He P, Hidalgo M, Baker SD. Differential metabolism of gefitinib and erlotinib by human cytochrome P450 enzymes. Clin Cancer Res. 2007. 13:3731–3737.23. Fan WC, Yu CJ, Tsai CM, Huang MS, Lai CL, Hsia TC, et al. Different efficacies of erlotinib and gefitinib in taiwanese patients with advanced non-small cell lung cancer: a retrospective multicenter study. J Thorac Oncol. 2011. 6:148–155.24. Wu YL, Zhong WZ, Li LY, Zhang XT, Zhang L, Zhou CC, et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol. 2007. 2:430–439.25. Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008. 372:1809–1818.26. Emery IF, Battelli C, Auclair PL, Carrier K, Hayes DM. Response to gefitinib and erlotinib in Non-small cell lung cancer: a restrospective study. BMC Cancer. 2009. 9:333.27. Maruyama R, Nishiwaki Y, Tamura T, Yamamoto N, Tsuboi M, Nakagawa K, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol. 2008. 26:4244–4252.28. Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006. 24:2549–2556.29. Inoue A, Saijo Y, Maemondo M, Gomi K, Tokue Y, Kimura Y, et al. Severe acute interstitial pneumonia and gefitinib. Lancet. 2003. 361:137–139.30. Hotta K, Kiura K, Takigawa N, Yoshioka H, Harita S, Kuyama S, et al. Comparison of the incidence and pattern of interstitial lung disease during erlotinib and gefitinib treatment in Japanese Patients with non-small cell lung cancer: the Okayama Lung Cancer Study Group experience. J Thorac Oncol. 2010. 5:179–184.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the therapeutic outcome between gefitinib and erlotinib in female patients with non-small-cell lung cancer
- Efficacy and Safety of Afatinib for EGFR-mutant Non-small Cell Lung Cancer, Compared with Gefitinib or Erlotinib
- Successful Rechallenge with Gefitinib for an Initial Erlotinib-Responder with Advanced Lung Adenocarcinoma
- Sequential Responses of Adenocarcinoma of the Lung to Erlotinib after Gefitinib in Never Smoker Korean Woman
- Comparison of Gefitinib and Erlotinib for Patients with Advanced Non-Small-Cell Lung Cancer