Tuberc Respir Dis.
2007 May;62(5):432-436.
Dramatic Tumor Response to 2nd-line Pemetrexed/Cisplatin Combination Chemotherapy in Patient with Malignant Pleural Mesothelioma.
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Konkuk University, Seoul, Korea. kyleemd@kuh.ac.kr
- 2Department of Radiology, College of Medicine, Konkuk University, Seoul, Korea.
- 3Department of Pathology, College of Medicine, Konkuk University, Seoul, Korea.
Abstract
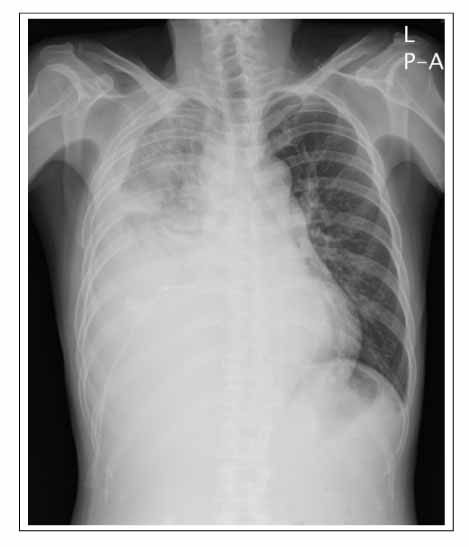
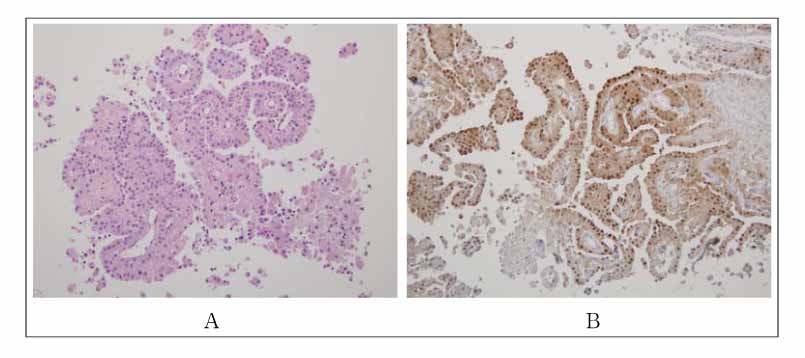
- Malignant pleural mesothelioma (MPM) is a rare tumor that is difficult to clearly distinguish from an adenocarcinoma but usually has a poor prognosis. Numerous cytotoxic agents have been used in the primary treatment of MPM with limited success. A complete response is unusual and a partial response occurs in less than one-third of patients. Recently, a phase III trial showed that a combination of pemetrexed with cisplatin resulted in a significantly higher response rate and median survival time than with cisplatin alone. We encountered a case of a dramatic tumor response to pemetrexed/cisplatin combination chemotherapy in patients with MPM, which was resistant to the 1st-line gemcitabine/cisplatin therapy. After six cycles of pemetrexed/cisplatin combination chemotherapy, the tumor volume had decreased dramatically with complete symptom relief. There was no chemotherapy-related toxicity or scheduled violation. The patient is under maintenance chemotherapy with the same regimen.
MeSH Terms
Figure
Reference
-
1. Ordonez NG. The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am J Surg Pathol. 2003. 27:1031–1051.2. Moskal TL, Urschel JD, Anderson TM, Antkowiak JG, Takita H. Malignant pleural mesothelioma: a problematic review. Surg Oncol. 1998. 7:5–12.3. Boutin C, Schlesser M, Frenay C, Astoul P. Malignant pleural mesothelioma. Eur Respir J. 1998. 12:972–981.4. Rusch VW, Venkatraman E. The importance of surgical staging in the treatment of malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 1996. 111:15–25.5. Ong ST, Vogelzang NJ. Chemotherapy in malignant pleural mesothelioma: a review. J Clin Oncol. 1996. 4:1007–1017.6. Ryan CW, Herndon J, Vogelzang NJ. A review of chemotherapy trials for malignant mesothelioma. Chest. 1998. 113:66S–73S.7. Alberts AS, Falkson G, Goedhals L, Vorobiof DA, Van der Merwe CA. Malignant pleural mesothelioma: a disease unaffected by current therapeutic maneuvers. J Clin Oncol. 1988. 6:527–535.8. Sugarbaker DJ, Heher EC, Lee TH, Couper G, Mentzer S, Corson JM, et al. Extrapleural pneumonectomy, chemotherapy and radiotherapy in the treatment of diffuse malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 1991. 102:10–15.9. Ball DL, Cruickshank DG. The treatment of malignant mesothelioma of the pleura: review of five-year experience, with special reference to radiotherapy. Am J Clin Oncol. 1990. 13:4–9.10. Allen KB, Faber LP, Warren WH. Malignant pleural mesothelioma: extrapleural pneumonectomy and pleurectomy. Chest Surg Clin N Am. 1994. 4:113–126.11. Rusthoven JJ, Eisenhauer E, Butts C, Gregg R, Dancey J, Fisher B, et al. Multitargeted antifolate LY231514 as first-line chemotherapy for patients with advanced non-small-cell lung cancer: a phase II study. J Clin Oncol. 1999. 17:1194.12. John W, Picus J, Blanke CD, Clark JW, Schulman LN, Rowinsky EK, et al. Activity of multitargeted antifolate (pemetrexed disodium, LY231514) in patients with advanced colorectal carcinoma: results from a phase II study. Cancer. 2000. 88:1807–1813.13. Miles DW, Smith IE, Coleman RE, Calvert AH, Lind MJ. A phase II study of pemetrexed disodium (LY231514) in patients with locally recurrent or metastatic breast cancer. Eur J Cancer. 2001. 37:366–371.14. Thodtmann R, Depenbrock H, Blatter J, Johnson RD, van Oosterom A, Hanauske AR, et al. Clinical and pharmacokinetic phase I study of multitargeted antifolate (LY 231514) in combination with cisplatin. J Clin Oncol. 1999. 17:3009–3016.15. Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003. 21:2636–2644.16. Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004. 15:257–260.17. Mendelsohn LG, Shih C, Chen VJ, Habeck LL, Gates SB, Shackelford KA. Enzyme inhibition, polyglutamation and the effect of LY231514 (MTA) on purine biosynthesis. Semin Oncol. 1999. 26:42–47.18. Schultz RM, Chen VJ, Bewley JR, Roberts EF, Shih C, Dempsey JA. Biological activity of the multitargeted antifolate, MTA (LY231514), in human cell lines with different resistance mechanisms to antifolate drugs. Semin Oncol. 1999. 26:68–73.19. Scagliotti GV, Shin DM, Kindler HL, Vasconcelles MJ, Keppler U, Manegold C, et al. Phase II study of pemetrexed with and without folic acid and vitamin B12 as front-line therapy in malignant pleural mesothelioma. J Clin Oncol. 2003. 21:1556–1561.20. Monetti F, Casanova S, Grasso A, Cafferata MA, Ardizzoni A, Neumaier CE. Inadequacy of the new response evaluation criteria in solid tumors (RECIST) in patients with malignant pleural mesothelioma: report of four cases. Lung Cancer. 2004. 43:71–74.21. Van Klaveren RJ, Aerts JG, de Bruin H, Giaccone G, Manegold C, Van Meerbeeck JP. Inadequacy of the RECIST criteria for response evaluation in patients with malignant pleural mesothelioma. Lung Cancer. 2004. 43:63–69.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Advanced Malignant Pleural Mesothelioma Treatment with Chemotherapy and Photodynamic Therapy
- A Case Report of Primary Pericardial Malignant Mesothelioma Treated with Pemetrexed and Cisplatin
- A Case of Diffuse Malignant Mesothelioma of the Peritoneum
- Chemotherapy in Advanced Urothelial Carcinoma
- Malignant Mesothelioma Causing Bloody Pleural Effusion