Korean J Urol.
2011 Jun;52(6):396-400.
Effect of Desmopressin with Anticholinergics in Female Patients with Overactive Bladder
- Affiliations
-
- 1Department of Urology, School of Medicine, Hallym University, Seoul, Korea. hykim@hallym.or.kr
Abstract
- PURPOSE
The aim of this study was to evaluate the effect of desmopressin combined with anticholinergics on daytime frequency and urgency in female patients with overactive bladder (OAB).
MATERIALS AND METHODS
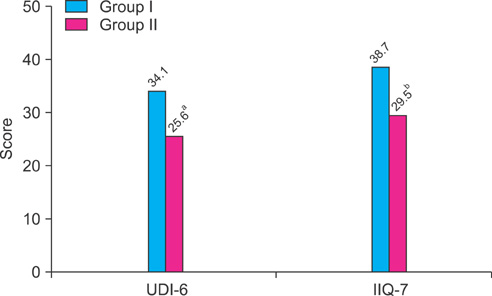
We included 68 female patients with OAB. Patients were randomly assigned to receive 5 mg of solifenacin (group I) or 5 mg of solifenacin and 0.2 mg of desmopressin (group II) for 2 weeks. A pre/post-treatment 3-day voiding diary and the Urinary Distress Inventory (UDI-6) and Incontinence Impact Questionnaire (IIQ-7) were used to assess changes in voiding symptoms and quality of life (QoL); results were compared between the two groups.
RESULTS
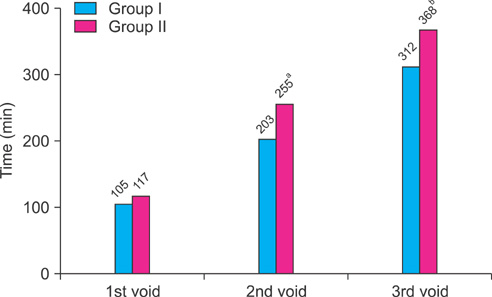
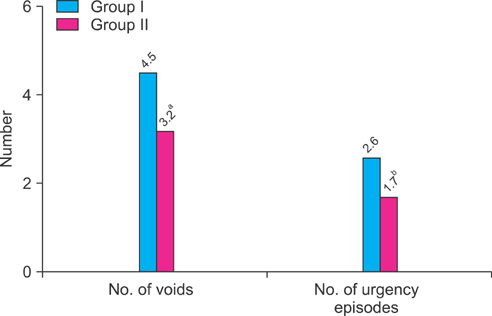
Groups I and II included 31 and 37 patients, respectively. Time to first void was 12 min later in group II (105 min vs. 117 min), but this difference was not statistically significant. However, time to the second and third voids (203 min vs. 255 min, 312 min vs. 368 min) and the first urgency episode (212 min vs. 255 min) were significantly longer in group II. Compared with group I, patients in group II showed significant improvement in QoL scores. When improvement after treatment was defined as increase in time to first void of greater than 10% after 2 weeks of treatment, desmopressin with anticholinergics was more effective in patients over the age of 65 years and with more than 150 ml of voided volume.
CONCLUSIONS
Desmopressin combined with anticholinergics was more effective than anticholinergics only in the treatment of female patients with OAB.
Keyword
MeSH Terms
Figure
Reference
-
1. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002. 21:167–178.2. Komaroff AL, Fagioli LR, Doolittle TH, Gandek B, Gleit MA, Guerriero RT, et al. Health status in patients with chronic fatigue syndrome and in general population and disease comparison groups. Am J Med. 1996. 101:281–290.3. Kelleher CJ, Reese PR, Pleil AM, Okano GJ. Health-related quality of life of patients receiving extended-release tolterodine for overactive bladder. Am J Manag Care. 2002. 8:19 Suppl. S608–S615.4. Liberman JN, Hunt TL, Stewart WF, Wein A, Zhou Z, Herzog AR, et al. Health-related quality of life among adults with symptoms of overactive bladder: results from a U.S. community-based survey. Urology. 2001. 57:1044–1050.5. Song HD, Cho SY, Lee KC, Cho IR. The risk factors for increased post-voiding residual urine volume after long-term anticholinergic therapy in patients with benign prostatic hyperplasia and overactive bladder. Korean J Urol. 2009. 50:982–988.6. Lee KS, Lee YS. Overactive bladder. Korean J Urol. 2007. 48:1191–1208.7. Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A medium-term analysis of the subjective efficacy of treatment for women with detrusor instability and low bladder compliance. Br J Obstet Gynaecol. 1997. 104:988–993.8. Andersson KE. Current concepts in the treatment of disorders of micturition. Drugs. 1988. 35:477–494.9. Nevéus T, Läckgren G, Tuvemo T, Olsson U, Stenberg A. Desmopressin resistant enuresis: pathogenetic and therapeutic considerations. J Urol. 1999. 162:2136–2140.10. Hashim H, Malmberg L, Graugaard-Jensen C, Abrams P. Desmopressin, as a "designer-drug," in the treatment of overactive bladder syndrome. Neurourol Urodyn. 2009. 28:40–46.11. Robinson D, Cardozo L, Akeson M, Hvistendahl G, Riis A, Norgaard JP. Antidiuresis: a new concept in managing female daytime urinary incontinence. BJU Int. 2004. 93:996–1000.12. Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Continence Program for Women Research Group. Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Neurourol Urodyn. 1995. 14:131–139.13. Asplund R, Sundberg B, Bengtsson P. Oral desmopressin for nocturnal polyuria in elderly subjects: a double-blind, placebo-controlled randomized exploratory study. BJU Int. 1999. 83:591–595.14. Mattiasson A, Abrams P, Van Kerrebroeck P, Walter S, Weiss J. Efficacy of desmopressin in the treatment of nocturia: a double-blind placebo-controlled study in men. BJU Int. 2002. 89:855–862.15. Lose G, Lalos O, Freeman RM, van Kerrebroeck P. Efficacy of desmopressin (Minirin) in the treatment of nocturia: a double-blind placebo-controlled study in women. Am J Obstet Gynecol. 2003. 189:1106–1113.16. van Kerrebroeck P, Rezapour M, Cortesse A, Thüroff J, Riis A, Nørgaard JP. Desmopressin in the treatment of nocturia: a double-blind, placebo-controlled study. Eur Urol. 2007. 52:221–229.17. Committee for Establishment of the Clinical Guidelines for Nocturia of the Neurogenic Bladder Society. Clinical guidelines for nocturia. Int J Urol. 2010. 17:397–409.18. Rembratt A, Riis A, Norgaard JP. Desmopressin treatment in nocturia; an analysis of risk factors for hyponatremia. Neurourol Urodyn. 2006. 25:105–109.19. Rembratt A, Graugaard-Jensen C, Senderovitz T, Norgaard JP, Djurhus JC. Pharmacokinetics and pharmacodynamics of desmopressin administered orally versus intravenously at daytime versus night-time in healthy men aged 55-70 years. Eur J Clin Pharmacol. 2004. 60:397–402.20. Weatherall M. The risk of hyponatremia in older adults using desmopressin for nocturia: a systematic review and meta-analysis. Neurourol Urodyn. 2004. 23:302–305.21. Mattiasson A, Abrams P, van Kerrebroeck P, Walters S, Weiss J. Efficacy of desmopressin in the treatment of nocturia: a double-blind placebo-controlled study in men. BJU Int. 2002. 89:855–862.22. Lose G, Lalos O, Freeman RM, van Kerrebroeck P. Efficacy of desmopressin (Minirin) in the treatment of nocturia: a double-blind placebo-controlled study in women. Am J Obstet Gynecol. 2003. 189:1106–1113.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Time Course of Treatment for Primary Enuresis With Overactive Bladder
- Pharmacological therapy of nocturnal enuresis
- Clinical Efficacy in the Treatment of Overactive Bladder Refractory to Anticholinergics by Posterior Tibial Nerve Stimulation
- Review of the Anticholinergics for the Treatment of Overactive Bladder: 2009 Update
- Influence of Type of Nocturia and Lower Urinary Tract Symptoms on Therapeutic Outcome in Women Treated With Desmopressin