Korean J Urol.
2009 Nov;50(11):1066-1072.
Early Experience of Prostate Cancer Treated with CyberKnife(TM) Radiotherapy
- Affiliations
-
- 1Department of Urology, College of Medicine, Konyang University, Daejeon, Korea. ovalboy@hanmail.net
- 2Department of Radiation Oncology, College of Medicine, Konyang University, Daejeon, Korea.
- 3Department of Urology, Chungnam University School of Medicine, Daejeon, Korea.
Abstract
- PURPOSE
We investigated the outcome in patients with prostatic cancer treated by means of CyberKnife(TM) radiotherapy.
MATERIALS AND METHODS
Between July 2007 and April 2009, 16 patients with prostate cancer underwent CyberKnife(TM) radiotherapy. The histologic diagnosis was established by transrectal ultrasonography-guided biopsy. Radiotherapy was performed for a dose of 34 Gy at 8.5 Gy per day over 4 to 18 days. Nine patients were treated with hormone therapy. After treatment, prostate-specific antigen (PSA) relapse was evaluated with periodic PSA follow-up.
RESULTS
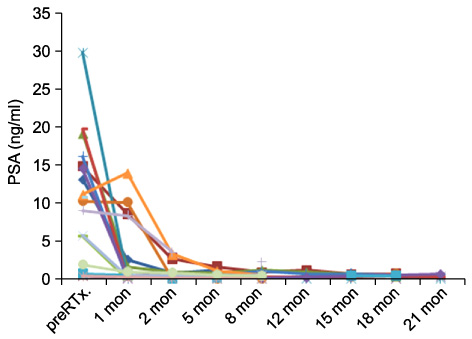
The numbers of patients in clinical stages T2 and T3 were 13 and 3, respectively. Two patients had lymph node metastasis with no distant metastasis. The numbers of patients with a Gleason grade of 5, 6, 7, 8, and 9 were 1, 5, 4, 3, and 2, respectively. The mean time to PSA nadir and the mean PSA at nadir were 7 months and 0.43 ng/ml, respectively. To date, there has been no biochemical failure or clinical recurrence. No severe complications were observed in any patients; observed minor complications [n (%)] were perianal pain [2 (12.5%)] and defecation discomfort [2 (12.5%)]. CONCLUSIONS: Generally good responses were observed in patients treated with CyberKnife(TM) radiotherapy for prostate cancer. No severe complications were observed. More patients and a longer follow-up are required for further conclusions.
MeSH Terms
Figure
Reference
-
1. Annual report of the Korea central cancer registry 2005. 2008. ministry of health and wealfare republic of Korea.2. Jani AB, Hellman S. Early prostate cancer: clinical decision-making. Lancet. 2003. 361:1045–1053.3. Roach M 3rd, DeSilvio M, Lawton C, Uhl V, Machtay M, Seider MJ, et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol. 2003. 21:1904–1911.4. Hyun JH, Chai SE, Choi HY. Predictors of biochemical failure after radical perineal prostatectomy for localized prostate cancer. Korean J Urol. 2003. 44:759–764.5. American Society for Therapeutic Radiology and Oncology Consensus Panel. Consensus statement: guidelines for PSA following radiation therapy. Int J Radiat Oncol Biol Phys. 1997. 37:1035–1041.6. D'Amico AV, Whittington R, Kaplan I, Beard C, Jiroutek M, Malkowicz SB, et al. Equivalent biochemical failure-free survival after external beam radiation therapy or radical prostatectomy in patients with a pretreatment prostate specific antigen of >4-20 ng/ml. Int J Radiat Oncol Biol Phys. 1997. 37:1053–1058.7. Kupelian PA, Elshaikh M, Reddy CA, Zippe C, Klein EA. Comparison of the efficacy of local therapies for localized prostate cancer in the prostate-specific antigen era: a large single-institution experience with radical prostatectomy and external-beam radiotherapy. J Clin Oncol. 2002. 20:3376–3385.8. Kim BK, Park SW, Ha SW. Pattern of decrease of prostate specific antigen after radical radiotherapy for the prostate cancer. J Korean Soc Ther Radiol Oncol. 1999. 17:136–140.9. Chun HC, Lee MZ. Radical radiotherapy for carcinoma of the prostate. J Korean Soc Ther Radiol Oncol. 2001. 19:40–44.10. Chin OH, Kim SI, Hong SJ. Early experience of localized prostate cancer treated with neoadjuvant androgen ablation therapy and radiotherapy. Korean J Urol. 2001. 42:702–706.11. Shimizu S, Shirato H, Kitamura K, Shinohara N, Harabayashi T, Tsukamoto T, et al. Use of an implanted marker and real-time tracking of the marker for the positioning of prostate and bladder cancers. Int J Radiat Oncol Biol Phys. 2000. 48:1591–1597.12. King CR, Lehmann J, Adler JR, Hai J. CyberKnife radiotherapy for localized prostate cancer: rationale and technical feasibility. Technol Cancer Res Treat. 2003. 2:25–30.13. Lawton CA, Won M, Pilepich MV, Asbell SO, Shipley WU, Hanks GE, et al. Long-term treatment sequelae following external beam irradiation for adenocarcinoma of the prostate: analysis of RTOG studies 7506 and 7706. Int J Radiat Oncol Biol Phys. 1991. 21:935–939.14. Toledano A, Chiche R, Lamallem H, Kanoui A, Beley S, Thibault F, et al. Elevation of PSA after prostate radiotherapy: rebound or biochemical recurrence? Prog Urol. 2008. 18:557–561.15. Partin AW, Oesterling JE. The clinical usefulness of prostate specific antigen: update 1994. J Urol. 1994. 152:1358–1368.16. Frazier HA, Robertson JE, Humphrey PA, Paulson DF. Is prostate specific antigen of clinical importance in evaluating outcome after radical prostatectomy? J Urol. 1993. 149:516–518.17. Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarinoma of the prostate. N Engl J Med. 1987. 317:909–916.18. Kim GY, Kim SI, Woo YN. Clincal course after external beam radiation therapy for localized prostate cancer. Korean J Urol. 2004. 45:1224–1228.19. Duchesne GM, Peters LJ. What is the alpha/beta ratio for prostate cancer? Rationale for hypofractionated high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 1999. 44:747–748.20. Dasu A. Is the alpha/beta value for prostate tumours low enough to be safely used in clinical trials? Clin Oncol. 2007. 19:289–301.21. Madsen BL, Hsi RA, Pham HT, Fowler JF, Esagui L, Corman J. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized prostate disease: first clinical trial results. Int J Radiat Oncol Biol Phys. 2007. 67:1099–1105.22. Demanes DJ, Rodriguez RR, Schour L, Brandt D, Altieri G. High-dose rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy's 10-year results. Int J Radiat Oncol Biol Phys. 2005. 61:1306–1316.23. Martinez A, Gonzalez J, Spencer W, Gustafson G, Kestin L, Kearney D, et al. Conformal high dose rate brachytherapy improves biochemical control and cause specific survival in patients with prostate cancer and poor prognostic factors. J Urol. 2003. 169:974–979.24. Fuller DB, Naitoh J, Lee C, Hardy S, Jin H. Virtual HDR Cyberknife treatment for localized prostatic carcinoma: dosimetry comparison with HDR brachytherapy and preliminary clinical observations. Int J Radiat Oncol Biol Phys. 2008. 70:1588–1597.25. King CR, Brooks JD, Gill H, Pawlicki T, Cotrutz C, Presti JC Jr. Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys. 2009. 73:1043–1048.26. Lee SJ, Song K, Park JW, Gil MC, Jo MK. Cyberknife™ for the treatment of non-metastatic prostate cancer. Korean J Urol. 2009. 50:744–750.27. O'Brien PC, Franklin CI, Poulsen MG, Joseph DJ, Spry NS, Denham JW. Acute symptoms, not rectally administered sucralfate, predict for late radiation proctitis: longer term follow-up of a phase III trial-Trans-Tasman Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2002. 54:442–449.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CyberKnife(TM) for the Treatment of Non-Metastatic Prostate Cancer
- Clinical Outcomes of CyberKnife Radiotherapy in Prostate Cancer Patients: Short-term, Single-Center Experience
- Hypofractionated stereotactic body radiotherapy in low- and intermediate-risk prostate carcinoma
- An Experience of Cyberknife Treatment in Patients with Advanced Pancreaticobilliary Malignancy
- CyberKnife for the Treatment of Nonmetastatic Prostate Cancer: Preliminary Results