Nutr Res Pract.
2016 Feb;10(1):19-25. 10.4162/nrp.2016.10.1.19.
Protective effect of dietary chitosan on cadmium accumulation in rats
- Affiliations
-
- 1Department of Food and Nutrition, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 08826, Korea. shindm@snu.ac.kr
- 2Research institution of human ecology, Seoul National University,1 Gwanak-ro, Gwanak-gu, Seoul 08826, Korea.
- KMID: 2313896
- DOI: http://doi.org/10.4162/nrp.2016.10.1.19
Abstract
- BACKGROUND/OBJECTIVES
Cadmium is a toxic metal that is an occupational and environmental concern especially because of its human carcinogenicity; it induces serious adverse effects in various organs and tissues. Even low levels of exposure to cadmium could be harmful owing to its extremely long half-life in the body. Cadmium intoxication may be prevented by the consumption of dietary components that potentially reduce its accumulation in the body. Dietary chitosan is a polysaccharide derived from animal sources; it has been known for its ability to bind to divalent cations including cadmium, in addition to other beneficial effects including hypocholesterolemic and anticancer effects. Therefore, we aimed to investigate the role of dietary chitosan in reducing cadmium accumulation using an in vivo system.
MATERIALS/METHODS
Cadmium was administered orally at 2 mg (three times per week) to three groups of Sprague-Dawley rats: control, low-dose, and high-dose (0, 3, and 5%, respectively) chitosan diet groups for eight weeks. Cadmium accumulation, as well as tissue functional and histological changes, was determined.
RESULTS
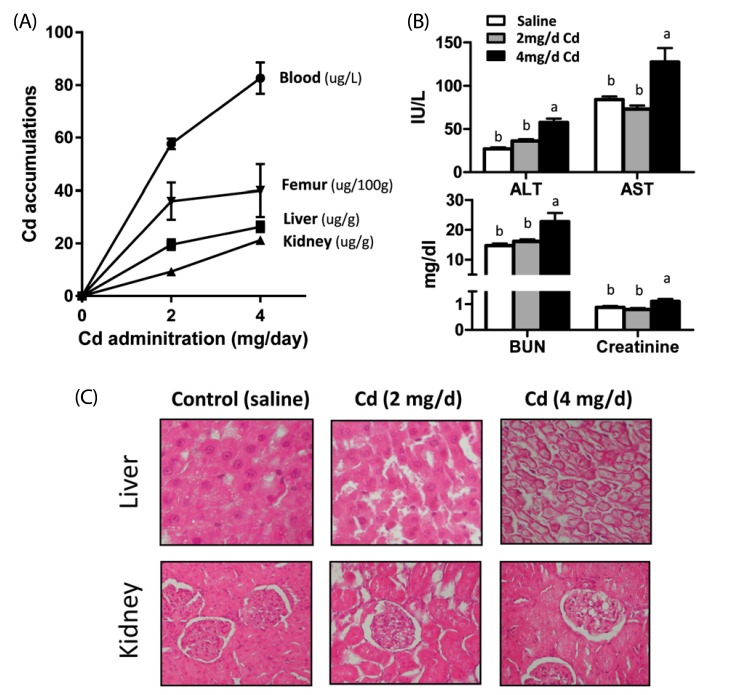
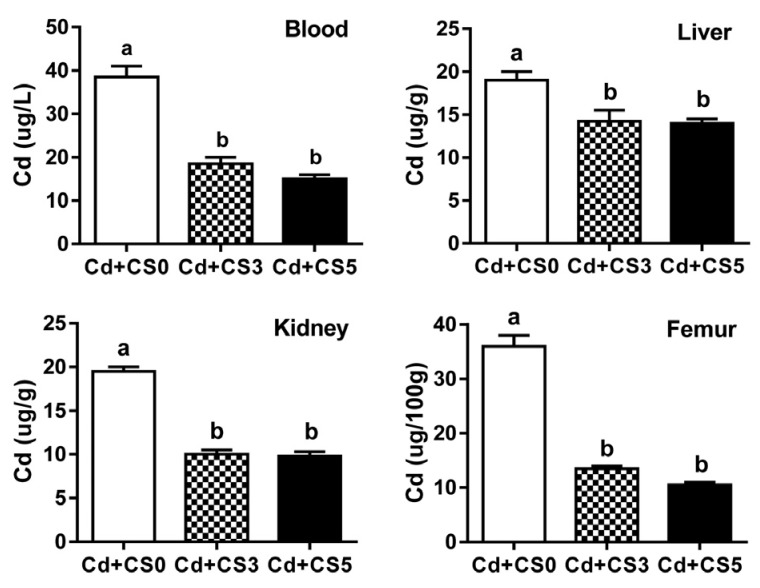
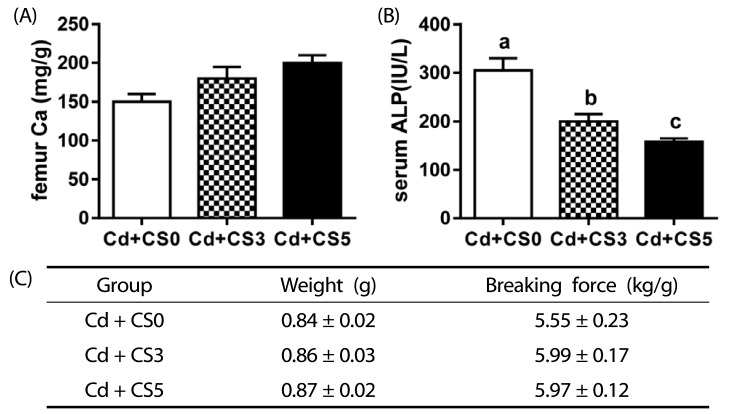
Compared to the control group, rats fed the chitosan diet showed significantly lower levels of cadmium in blood and tissues including the kidneys, liver, and femur. Biochemical analysis of liver function including the determination of aspartate aminotransferase and total bilirubin levels showed that dietary chitosan reduced hepatic tissue damage caused by cadmium intoxication and prevented the associated bone disorder.
CONCLUSIONS
These results suggest that dietary chitosan has the potential to reduce cadmium accumulation in the body as well as protect liver function and bone health against cadmium intoxication.
Keyword
MeSH Terms
Figure
Reference
-
1. Järup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009; 238:201–208. PMID: 19409405.
Article2. Clemens S, Aarts MG, Thomine S, Verbruggen N. Plant science: the key to preventing slow cadmium poisoning. Trends Plant Sci. 2013; 18:92–99. PMID: 22981394.
Article3. European Food Safety Authority. Cadmium dietary exposure in the European population. EFSA J. 2012; 10:2551.4. Sharma A. Evaluation of certain food additives and contaminants, Seventy-third report of the Joint FAO/WHO Expert Committee on Food Additives. Indian J Med Res. 2012; 135:446–447.5. Olsson IM, Bensryd I, Lundh T, Ottosson H, Skerfving S, Oskarsson A. Cadmium in blood and urine--impact of sex, age, dietary intake, iron status, and former smoking--association of renal effects. Environ Health Perspect. 2002; 110:1185–1190. PMID: 12460796.
Article6. Järup L, Rogenfelt A, Elinder CG, Nogawa K, Kjellström T. Biological half-time of cadmium in the blood of workers after cessation of exposure. Scand J Work Environ Health. 1983; 9:327–331. PMID: 6635611.
Article7. Adams SV, Passarelli MN, Newcomb PA. Cadmium exposure and cancer mortality in the Third National Health and Nutrition Examination Survey cohort. Occup Environ Med. 2012; 69:153–156. PMID: 22068173.8. Engström A, Michaëlsson K, Vahter M, Julin B, Wolk A, Åkesson A. Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women. Bone. 2012; 50:1372–1378. PMID: 22465267.
Article9. Nordberg G, Jin T, Wu X, Lu J, Chen L, Liang Y, Lei L, Hong F, Bergdahl IA, Nordberg M. Kidney dysfunction and cadmium exposure--factors influencing dose-response relationships. J Trace Elem Med Biol. 2012; 26:197–200. PMID: 22565016.10. Wu KC, Liu JJ, Klaassen CD. Nrf2 activation prevents cadmium-induced acute liver injury. Toxicol Appl Pharmacol. 2012; 263:14–20. PMID: 22677785.
Article11. Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure, and health outcomes. Cien Saude Colet. 2011; 16:2587–2602. PMID: 21655733.
Article12. Amamou F, Nemmiche S, Meziane RK, Didi A, Yazit SM, Chabane-Sari D. Protective effect of olive oil and colocynth oil against cadmiuminduced oxidative stress in the liver of Wistar rats. Food Chem Toxicol. 2015; 78:177–184. PMID: 25617810.
Article13. Thomas LD, Hodgson S, Nieuwenhuijsen M, Jarup L. Early kidney damage in a population exposed to cadmium and other heavy metals. Environ Health Perspect. 2009; 117:181–184. PMID: 19270785.
Article14. Noonan CW, Sarasua SM, Campagna D, Kathman SJ, Lybarger JA, Mueller PW. Effects of exposure to low levels of environmental cadmium on renal biomarkers. Environ Health Perspect. 2002; 110:151–155. PMID: 11836143.
Article15. de Burbure C, Buchet JP, Leroyer A, Nisse C, Haguenoer JM, Mutti A, Smerhovsky Z, Cikrt M, Trzcinka-Ochocka M, Razniewska G, Jakubowski M, Bernard A. Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels. Environ Health Perspect. 2006; 114:584–590. PMID: 16581550.
Article16. Kobayashi E, Suwazono Y, Dochi M, Honda R, Nishijo M, Kido T, Nakagawa H. Estimation of benchmark doses as threshold levels of urinary cadmium, based on excretion of β2-microglobulin in cadmium-polluted and non-polluted regions in Japan. Toxicol Lett. 2008; 179:108–112. PMID: 18515022.
Article17. Inaba T, Kobayashi E, Suwazono Y, Uetani M, Oishi M, Nakagawa H, Nogawa K. Estimation of cumulative cadmium intake causing Itai-itai disease. Toxicol Lett. 2005; 159:192–201. PMID: 16006079.
Article18. Gallagher CM, Kovach JS, Meliker JR. Urinary cadmium and osteoporosis in U.S. Women >or= 50 years of age: NHANES 1988-1994 and 1999-2004. Environ Health Perspect. 2008; 116:1338–1343. PMID: 18941575.19. Schutte R, Nawrot TS, Richart T, Thijs L, Vanderschueren D, Kuznetsova T, Van Hecke E, Roels HA, Staessen JA. Bone resorption and environmental exposure to cadmium in women: a population study. Environ Health Perspect. 2008; 116:777–783. PMID: 18560534.
Article20. Yuan G, Dai S, Yin Z, Lu H, Jia R, Xu J, Song X, Li L, Shu Y, Zhao X. Toxicological assessment of combined lead and cadmium: acute and sub-chronic toxicity study in rats. Food Chem Toxicol. 2014; 65:260–268. PMID: 24394482.
Article21. Eriksen KT, Halkjær J, Sørensen M, Meliker JR, McElroy JA, Tjønneland A, Raaschou-Nielsen O. Dietary cadmium intake and risk of breast, endometrial and ovarian cancer in Danish postmenopausal women: a prospective cohort study. PLoS One. 2014; 9:e100815. PMID: 24963789.
Article22. Harish Prashanth KV, Tharanathan RN. Chitin/chitosan: modifications and their unlimited application potential--an overview. Trends Food Sci Technol. 2007; 18:117–131.
Article23. Samarasinghe RM, Kanwar RK, Kanwar JR. The effect of oral administration of iron saturated-bovine lactoferrin encapsulated chitosan-nanocarriers on osteoarthritis. Biomaterials. 2014; 35:7522–7534. PMID: 24933511.
Article24. Zhang J, Liu J, Li L, Xia W. Dietary chitosan improves hypercholesterolemia in rats fed high-fat diets. Nutr Res. 2008; 28:383–390. PMID: 19083436.
Article25. Yamada S, Ganno T, Ohara N, Hayashi Y. Chitosan monomer accelerates alkaline phosphatase activity on human osteoblastic cells under hypofunctional conditions. J Biomed Mater Res A. 2007; 83:290–295. PMID: 17415763.
Article26. Liao FH, Shieh MJ, Chang NC, Chien YW. Chitosan supplementation lowers serum lipids and maintains normal calcium, magnesium, and iron status in hyperlipidemic patients. Nutr Res. 2007; 27:146–151.
Article27. Qin C, Du Y, Xiao L, Li Z, Gao X. Enzymic preparation of water-soluble chitosan and their antitumor activity. Int J Biol Macromol. 2002; 31:111–117. PMID: 12559434.
Article28. Gallaher DD, Gallaher CM, Mahrt GJ, Carr TP, Hollingshead CH, Hesslink R Jr, Wise J. A glucomannan and chitosan fiber supplement decreases plasma cholesterol and increases cholesterol excretion in overweight normocholesterolemic humans. J Am Coll Nutr. 2002; 21:428–433. PMID: 12356785.
Article29. Meininger CJ, Kelly KA, Li H, Haynes TE, Wu G. Glucosamine inhibits inducible nitric oxide synthesis. Biochem Biophys Res Commun. 2000; 279:234–239. PMID: 11112445.
Article30. Shen KT, Chen MH, Chan HY, Jeng JH, Wang YJ. Inhibitory effects of chitooligosaccharides on tumor growth and metastasis. Food Chem Toxicol. 2009; 47:1864–1871. PMID: 19427889.
Article31. Renault F, Sancey B, Badot PM, Crini G. Chitosan for coagulation/flocculation processes-an eco-friendly approach. Eur Polym J. 2009; 45:1337–1348.32. Gamage A, Shahidi F. Use of chitosan for the removal of metal ion contaminants and proteins from water. Food Chem. 2007; 104:989–996.
Article33. Bravo-Osuna I, Millotti G, Vauthier C, Ponchel G. In vitro evaluation of calcium binding capacity of chitosan and thiolated chitosan poly (isobutyl cyanoacrylate) core-shell nanoparticles. Int J Pharm. 2007; 338:284–290. PMID: 17367968.34. Lima IS, Airoldi C. Interaction of copper with chitosan and succinic anhydride derivative-a factorial design evaluation of the chemisorption process. Colloids Surf A Physicochem Eng Asp. 2003; 229:129–136.
Article35. Crini G, Badot PM. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci. 2008; 33:399–447.
Article37. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993; 123:1939–1951. PMID: 8229312.
Article38. Klaassen CD, Liu J, Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol Toxicol. 1999; 39:267–294. PMID: 10331085.
Article39. Bikle DD. Biochemical markers in the assessment of bone disease. Am J Med. 1997; 103:427–436. PMID: 9375712.
Article40. Nordberg GF, Nogawa K, Nordberg M, Friberg LT. Cadmium. In : Nordberg GF, Fowler BA, Nordberg M, Friberg LT, editors. Handbook on the Toxicology of Metals. 3rd ed. Amsterdam: Academic Press;2007. p. 446–486.41. Leazer TM, Liu Y, Klaassen CD. Cadmium absorption and its relationship to divalent metal transporter-1 in the pregnant rat. Toxicol Appl Pharmacol. 2002; 185:18–24. PMID: 12460733.
Article42. Alfvén T, Elinder CG, Carlsson MD, Grubb A, Hellström L, Persson B, Pettersson C, Spång G, Schütz A, Järup L. Low-level cadmium exposure and osteoporosis. J Bone Miner Res. 2000; 15:1579–1586. PMID: 10934657.
Article43. Staessen JA, Roels HA, Emelianov D, Kuznetsova T, Thijs L, Vangronsveld J, Fagard R. Public Health and Environmental Exposure to Cadmium (PheeCad) Study Group. Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Lancet. 1999; 353:1140–1144. PMID: 10209978.
Article44. Choi JH, Rhee IK, Park KY, Park KY, Kim JK, Rhee SJ. Action of green tea catechin on bone metabolic disorder in chronic cadmium-poisoned rats. Life Sci. 2003; 73:1479–1489. PMID: 12865088.
Article45. Chan EL, Swaminathan R. Calcium metabolism and bone calcium content in normal and oophorectomized rats consuming various levels of saline for 12 months. J Nutr. 1998; 128:633–639. PMID: 9482774.
Article46. Sims NA, Morris HA, Moore RJ, Durbridge TC. Parathyroidectomy does not prevent bone loss in the oophorectomized rat. J Bone Miner Res. 1994; 9:1859–1863. PMID: 7872050.
Article47. Shim JA, Son YA, Park JM, Kim MK. Effect of Chlorella intake on Cadmium metabolism in rats. Nutr Res Pract. 2009; 3:15–22. PMID: 20016697.
Article48. Kim YJ, Kwon S, Kim MK. Effect of Chlorella vulgaris intake on cadmium detoxification in rats fed cadmium. Nutr Res Pract. 2009; 3:89–94. PMID: 20016707.
Article49. Je JY, Kim SK. Reactive oxygen species scavenging activity of aminoderivatized chitosan with different degree of deacetylation. Bioorg Med Chem. 2006; 14:5989–5994. PMID: 16725329.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A study on the effect of garlic to the heavy metal poisoning of rat
- Effect of Vitamin E on Cadmium Accumulation and Excretion in Chronic Cadmium Poisoned Rats
- Changes of Renal Cadmium Accumulation Levels and Urinary Cadmium Excretion Levels Induced by Long-term Cadmium Exposure in Rats
- Morphological change of the olfactory epithelium of rats in cadmium poisoning
- Effect of Chlorella intake on Cadmium metabolism in rats