Nutr Res Pract.
2015 Dec;9(6):599-605. 10.4162/nrp.2015.9.6.599.
Bioconversion of Citrus unshiu peel extracts with cytolase suppresses adipogenic activity in 3T3-L1 cells
- Affiliations
-
- 1Department of Food and Nutrition, College of Natural Sciences, Myongji University, 116 Myongji-ro, Cheoin-gu, Yongin, Gyeonggi 449-728, Korea. jhwang@mju.ac.kr
- 2BK Bio Co. Ltd., Gyeonggi 462-819, Korea.
- KMID: 2313883
- DOI: http://doi.org/10.4162/nrp.2015.9.6.599
Abstract
- BACKGROUND/OBJECTIVES
Citrus flavonoids have a variety of physiological properties such as anti-oxidant, anti-inflammation, anti-cancer, and anti-obesity. We investigated whether bioconversion of Citrus unshiu with cytolase (CU-C) ameliorates the anti-adipogenic effects by modulation of adipocyte differentiation and lipid metabolism in 3T3-L1 cells.
MATERIALS/METHODS
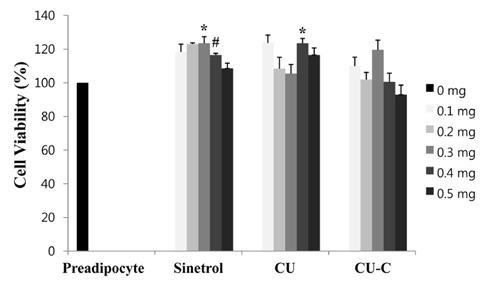
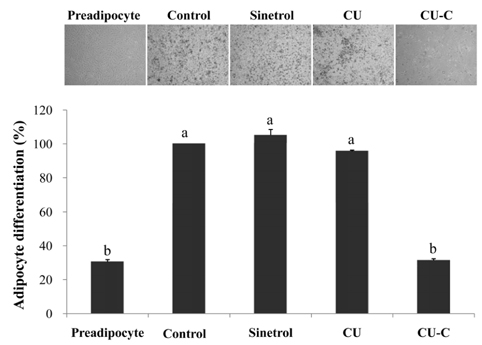
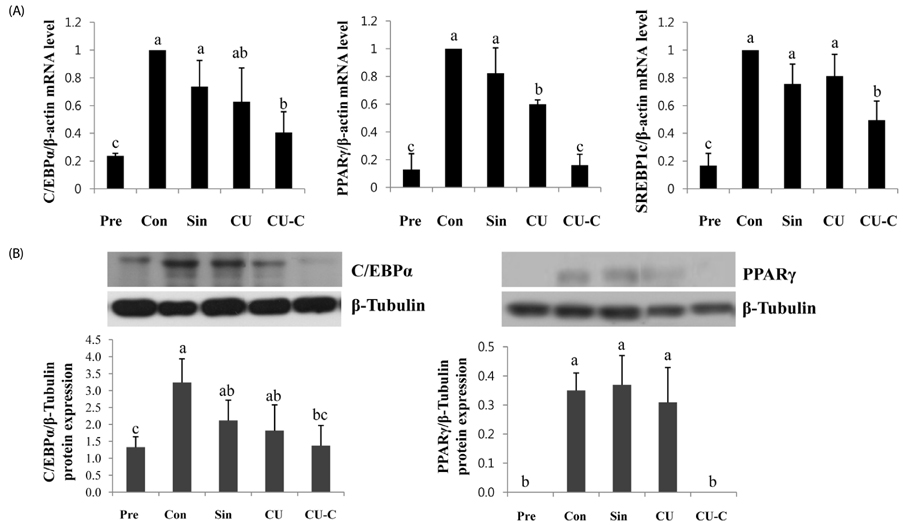
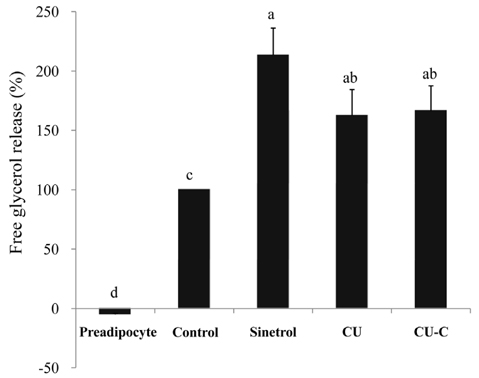
Glycoside forms of Citrus unshiu (CU) were converted into aglycoside forms with cytolase treatment. Cell viability of CU and CU-C was measured at various concentrations in 3T3L-1 cells. The anti-adipogenic and lipolytic effects were examined using Oil red O staining and free glycerol assay, respectively. We performed real time-polymerase chain reaction and western immunoblotting assay to detect mRNA and protein expression of adipogenic transcription factors, respectively.
RESULTS
Treatment with cytolase decreased flavanone rutinoside forms (narirutin and hesperidin) and instead, increased flavanone aglycoside forms (naringenin and hesperetin). During adipocyte differentiation, 3T3-L1 cells were treated with CU or CU-C at a dose of 0.5 mg/ml. Adipocyte differentiation was inhibited in CU-C group, but not in CU group. CU-C markedly suppressed the insulin-induced protein expression of CCAAT/enhancer-binding protein alpha (C/EBPalpha) and peroxisome proliferator-activated receptor gamma (PPARgamma) as well as the mRNA levels of CEBPalpha, PPARgamma, and sterol regulatory element binding protein 1c (SREBP1c). Both CU and CU-C groups significantly increased the adipolytic activity with the higher release of free glycerol than those of control group in differentiated 3T3-L1 adipocytes. CU-C is particularly superior in suppression of adipogenesis, whereas CU-C has similar effect to CU on stimulation of lipolysis.
CONCLUSIONS
These results suggest that bioconversion of Citrus unshiu peel extracts with cytolase enhances aglycoside flavonoids and improves the anti-adipogenic metabolism via both inhibition of key adipogenic transcription factors and induction of adipolytic activity.
Keyword
MeSH Terms
-
3T3-L1 Cells*
Adipocytes
Adipogenesis
Blotting, Western
Cell Survival
Citrus*
Flavonoids
Glycerol
Lipid Metabolism
Lipolysis
Metabolism
PPAR gamma
RNA, Messenger
Sterol Regulatory Element Binding Protein 1
Transcription Factors
Flavonoids
Glycerol
PPAR gamma
RNA, Messenger
Sterol Regulatory Element Binding Protein 1
Transcription Factors
Figure
Reference
-
1. Kopelman PG. Obesity as a medical problem. Nature. 2000; 404:635–643.
Article2. Ailhaud G, Guesnet P, Cunnane SC. An emerging risk factor for obesity: does disequilibrium of polyunsaturated fatty acid metabolism contribute to excessive adipose tissue development? Br J Nutr. 2008; 100:461–470.
Article3. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004; 89:2548–2556.
Article4. Park HS, Kim SH, Kim YS, Ryu SY, Hwang JT, Yang HJ, Kim GH, Kwon DY, Kim MS. Luteolin inhibits adipogenic differentiation by regulating PPARγ activation. Biofactors. 2009; 35:373–379.
Article5. Farmer SR. Regulation of PPARgamma activity during adipogenesis. Int J Obes (Lond). 2005; 29:Suppl 1. S13–S16.6. Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006; 4:263–273.
Article7. Zuo Y, Qiang L, Farmer SR. Activation of CCAAT/enhancer-binding protein (C/EBP) α expression by C/EBP β during adipogenesis requires a peroxisome proliferator-activated receptor-γ-associated repression of HDAC1 at the C/EBP α gene promoter. J Biol Chem. 2006; 281:7960–7967.
Article8. Wang X, Briggs MR, Hua X, Yokoyama C, Goldstein JL, Brown MS. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter. II. Purification and characterization. J Biol Chem. 1993; 268:14497–14504.
Article9. Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996; 10:1096–1107.
Article10. Ejaz S, Ejaz A, Matsuda K, Lim CW. Limonoids as cancer chemopreventive agents. J Sci Food Agric. 2006; 86:339–345.
Article11. Kang SI, Shin HS, Kim HM, Hong YS, Yoon SA, Kang SW, Kim JH, Kim MH, Ko HC, Kim SJ. Immature Citrus sunki peel extract exhibits antiobesity effects by β-oxidation and lipolysis in high-fat diet-induced obese mice. Biol Pharm Bull. 2012; 35:223–230.
Article12. Nichols LA, Jackson DE, Manthey JA, Shukla SD, Holland LJ. Citrus flavonoids repress the mRNA for stearoyl-CoA desaturase, a key enzyme in lipid synthesis and obesity control, in rat primary hepatocytes. Lipids Health Dis. 2011; 10:36–40.
Article13. Peterson J, Dwyer J. Flavonoids: dietary occurrence and biochemical activity. Nutr Res. 1998; 18:1995–2018.
Article14. Tripoli E, Guardia ML, Giammanco S, Majo DD, Giammanco M. Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem. 2007; 104:466–479.
Article15. Fuhr U, Kummert AL. The fate of naringin in humans: a key to grapefruit juice-drug interactions? Clin Pharmacol Ther. 1995; 58:365–373.
Article16. Seo JY, Lee JH, Kim NW, Her E, Chang SH, Ko NY, Yoo YH, Kim JW, Seo DW, Han JW, Kim YM, Choi WS. Effect of a fermented ginseng extract, BST204, on the expression of cyclooxygenase-2 in murine macrophages. Int Immunopharmacol. 2005; 5:929–936.
Article17. Yang G, Park D, Lee J, Song BS, Jeon TH, Kang SJ, Jeon JH, Shin S, Jeong H, Lee H, Kim Y. Suppressive effects of red ginseng preparations on SW480 colon cancer xenografts in mice. Food Sci Biotechnol. 2011; 20:1649–1653.
Article18. Dallas C, Gerbi A, Tenca G, Juchaux F, Bernard FX. Lipolytic effect of a polyphenolic citrus dry extract of red orange, grapefruit, orange (SINETROL) in human body fat adipocytes. Mechanism of action by inhibition of cAMP-phosphodiesterase (PDE). Phytomedicine. 2008; 15:783–792.
Article19. Kim GS, Park HJ, Woo JH, Kim MK, Koh PO, Min W, Ko YG, Kim CH, Won CK, Cho JH. Citrus aurantium flavonoids inhibit adipogenesis through the Akt signaling pathway in 3T3-L1 cells. BMC Complement Altern Med. 2012; 12:31.
Article20. Rayalam S, Della-Fera MA, Baile CA. Phytochemicals and regulation of the adipocyte life cycle. J Nutr Biochem. 2008; 19:717–726.
Article21. Liu L, Shan S, Zhang K, Ning ZQ, Lu XP, Cheng YY. Naringenin and hesperetin, two flavonoids derived from Citrus aurantium up-regulate transcription of adiponectin. Phytother Res. 2008; 22:1400–1403.
Article22. Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004; 113:846–855.
Article23. Matsuda H, Kogami Y, Nakamura S, Sugiyama T, Ueno T, Yoshikawa M. Structural requirements of flavonoids for the adipogenesis of 3T3-L1 cells. Bioorg Med Chem. 2011; 19:2835–2841.
Article24. Haaz S, Fontaine KR, Cutter G, Limdi N, Perumean-Chaney S, Allison DB. Citrus aurantium and synephrine alkaloids in the treatment of overweight and obesity: an update. Obes Rev. 2006; 7:79–88.
Article25. Mauriège P, De Pergola G, Berlan M, Lafontan M. Human fat cell beta-adrenergic receptors: beta-agonist-dependent lipolytic responses and characterization of beta-adrenergic binding sites on human fat cell membranes with highly selective beta 1-antagonists. J Lipid Res. 1988; 29:587–601.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimization of the mixed ratio of organic tangerine peel and guarana extracts to suppress fat accumulation
- Bioconverted Jeju Hallabong tangor (Citrus kiyomi × ponkan) peel extracts by cytolase enhance antioxidant and anti-inflammatory capacity in RAW 264.7 cells
- Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation
- Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte
- Effects of Different Mandarin Formulations on Antioxidative Capacity and Oxidative DNA Damage in Fifteen-Month Aged Rats