Nutr Res Pract.
2013 Dec;7(6):466-474.
Fenugreek seeds reduce aluminum toxicity associated with renal failure in rats
- Affiliations
-
- 1Laboratory of Histology and Cytogenetic (Research unit of Genetic 02/UR/08-03), Faculty of Medicine, Avenue Ibnou Sina 5000 Monastir, Tunisia. yosrabelaid@gmail.com
Abstract
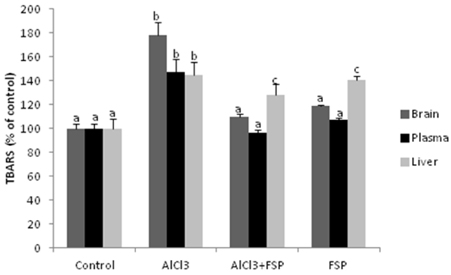
- Despite the reports on safety concerns regarding the relationship between aluminum salts and neurological and bone disease, many countries continue to use aluminum as phosphate binders among patients with renal failure. In search for a diet supplement that could reduce aluminum toxicity related to renal failure, we carried out this prospective animal study in which the fenugreek seeds were assessed for their effects on rats nephrotoxicity induced by aluminum chloride (AlCl3). Oral AlCl3 administration during 5 months (500 mg/kg bw i.g for one month then 1600 ppm via drinking water) led to plasma biochemical changes, an inhibition of alkaline phosphatase (ALP), a decrease of total antioxidant status (TAS), and an induction of lipid peroxidation (LPO) in the blood and brain, in addition to kidney atrophy and morphological alterations at the level of Bowman's capsule, the glomerulus and different sorts of tubules, reminiscent of some known kidney disease. The treatment with the whole fenugreek seed powder (FSP) (5% in the diet) during the last 2 months showed its effectiveness in restoring normal plasma values of urea, creatinine, ALP and glucose, as well as re-increasing the TAS, inhibiting LPO and alleviating histopathological changes in the injured kidneys. This study highlights the induced nephrotoxicicity, as well as the related toxicity in the brain and bone, by chronic oral ingestion of the aluminum salts. However, the maintenance of a diet supplemented with fenugreek seeds could offer protection for the kidney, bone and brain, at the same time.
Keyword
MeSH Terms
-
Alkaline Phosphatase
Aluminum Compounds
Aluminum*
Animals
Atrophy
Bone Diseases
Bowman Capsule
Brain
Chlorides
Creatinine
Diet
Drinking
Eating
Glucose
Humans
Kidney
Kidney Diseases
Lipid Peroxidation
Plasma
Prospective Studies
Rats*
Renal Insufficiency*
Salts
Trigonella*
Urea
Alkaline Phosphatase
Aluminum
Aluminum Compounds
Chlorides
Creatinine
Glucose
Salts
Urea
Figure
Reference
-
1. Nieboer E, Gibson BL, Oxman AD, Kramer JR. Health effects of aluminum: a critical review with emphasis on aluminum in drinking water. Environ Rev. 1995; 3(1):29–81.
Article2. Mudge DW, Johnson DW, Hawley CM, Campbell SB, Isbel NM, Van Eps CL, et al. Do aluminium-based phosphate binders continue to have a role in contemporary nephrology practice? BMC Nephrol. 2011; 12:20.
Article3. Laroubi A, Touhami M, Farouk L, Zrara I, Aboufatima R, Benharref A, et al. Prophylaxis effect of Trigonella foenum graecum L. seeds on renal stone formation in rats. Phytother Res. 2007; 21:921–925.
Article4. Satheeshkumar N, Mukherjee PK, Bhadra S, Saha BP. Acetylcholinesterase enzyme inhibitory potential of standardized extract of Trigonella foenum graecum L and its constituents. Phytomedicine. 2010; 17(3-4):292–295.
Article5. Sahin G, Varol I, Temizer A, Benli K, Demirdamar R, Duru S. Determination of aluminum levels in the kidney, liver, and brain of mice treated with aluminum hydroxide. Biol Trace Elem Res. 1994; 41(1-2):129–135.
Article6. Somova LI, Missankov A, Khan MS. Chronic aluminum intoxication in rats: dose-dependent morphological changes. Methods Find Exp Clin Pharmacol. 1997; 19(9):599–604.7. Bellakhdar J. La pharmacopee marocaine traditionnelle. Médecine arabe ancienne et savoirs populaires. Ibis Press;1998. p. 764.8. Wichtl M, Anton R. Plantes thérapeutiques. Strasgourg: Tec & Doc;1999. p. 636.9. Shang M, Cai S, Han J, Li J, Zhao Y, Zheng J, et al. Studies on flavonoids from Fenugreek (Trigonella foenumgraecum L.)]. Zhongguo Zhong Yao Za Zhi. 1998; 23(10):614–616. 63910. Gong QH, Wu Q, Huang XN, Sun AS, Shi JS. Protective effects of Ginkgo biloba leaf extract on aluminum-induced brain dysfunction in rats. Life Sci. 2005; 77(2):140–148.
Article11. Rao PU, Sesikeran B, Rao PS, Naidu AN, Rao VV, Ramachandran EP. Short term nutritional and safety evaluation of fenugreek. Nutr Res. 1996; 16(9):1495–1505.
Article12. Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynecol. 1979; 135(3):372–376.
Article13. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978; 52:302–310.14. Gabe M. Techniques Histologiques. Paris: Masson;1986.15. Pears A. Histochemistry, theoretical and applied. London: Churchill;1985.16. Exley C, Burgess E, Day JP, Jeffery EH, Melethil S, Yokel RA. Aluminum toxicokinetics. J Toxicol Environ Health. 1996; 48(6):569–584.
Article17. Stacchiotti A, Rodella LF, Ricci F, Rezzani R, Lavazza A, Bianchi R. Stress proteins expression in rat kidney and liver chronically exposed to aluminium sulphate. Histol Histopathol. 2006; 21(2):131–140.18. He Q, Heshka S, Albu J, Boxt L, Krasnow N, Elia M, et al. Smaller organ mass with greater age, except for heart. J Appl Physiol. 2009; 106(6):1780–1784.
Article19. Newairy AS, Salama AF, Hussien HM, Yousef MI. Propolis alleviates aluminium induced lipid peroxidation and biochemical parameters in male rats. Food Chem Toxicol. 2009; 47:1093–1098.
Article20. Whelton A, Watson AJ, Rock RC. Nitrogen Metabolites and Renal Function. In : Burtis C, Ashwood E, editors. Text book of Clinical Chemistry. Philadelphia: Saunders W.B.;1994. p. 1513–1575.21. Rao VH, Meehan DT, Delimont D, Nakajima M, Wada T, Gratton MA, et al. Role for macrophage metalloelastase in glomerular basement membrane damage associated with alport syndrome. Am J Pathol. 2006; 169(1):32–46.
Article22. Smeets B, Steenbergen MLM, Dijkman HBPM, Verrijp KN, Te Loeke NAJM, Aten J, et al. Angiotensin converting enzyme inhibition prevents development of collapsing focal segmental glomerulosclerosis in Thy-1.1 transgenic mice. Nephrol Dial Transplant. 2006; 21(11):3087–3097.
Article23. Sargazi M, Shenkin A, Roberts NB. Aluminium-induced injury to kidney proximal tubular cells: Effects on markers of oxidative damage. J Trace Elem Med Biol. 2006; 19(4):267–273.
Article24. Mahieu ST, Gionotti M, Millen N, Elías MM. Effect of chronic accumulation of aluminum on renal function, cortical renal oxidative stress and cortical renal organic anion transport in rats. Arch Toxicol. 2003; 77(11):605–612.
Article25. Thakran S, Siddiqui MR, Baquer NZ. Trigonella foenum graecum seed powder protects against histopathological abnormalities in tissues of diabetic rats. Mol Cell Biochem. 2004; 266(1-2):151–159.
Article26. Koyama I, Yakushijin M, Nakajima T, Hokari S, Kawai S, Oh-Ie K, et al. Reduced alkaline phosphatase activity in diabetic rat bone: a re-evaluation. Comp Biochem Physiol B Biochem Mol Biol. 1998; 121(4):417–423.
Article27. Whyte MP. Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev. 1994; 15(4):439–461.
Article28. Foldes J, Balena R, Ho A, Parfitt AM, Kleerekoper M. Hypophosphatemic rickets with hypocalciuria following long-term treatment with aluminum-containing antacid. Bone. 1991; 12(2):67–71.
Article29. Skillen AW, Hawthorne GC, Turner GA. Serum alkaline phosphatase in rats with streptozotocin-induced diabetes. Horm Metab Res. 1987; 19(10):505–506.
Article30. Miu AC, Benga O. Aluminum and Alzheimer's disease: a new look. J Alzheimers Dis. 2006; 10(2-3):179–201.
Article31. Li XB, Zhang ZY, Yin LH, Schluesener HJ. The profile of β-amyloid precursor protein expression of rats induced by aluminum. Environ Toxicol Pharmacol. 2012; 33(2):135–140.
Article32. Kovar I, Mayne P, Barltrop D. Plasma alkaline phosphatase activity: a screening test for rickets in preterm neonates. Lancet. 1982; 1(8267):308–310.
Article33. Sherif M, Awadallah R, Amrallah A. Determination of trace elements of Egyptian crops by neutron activation analysis. J Radioanal Nucl Chem. 1980; 57(1):53–60.
Article34. Yoshida H, Nagaya T, Hayashi T, Takahashi H, Kawai M. Milk consumption decreases activity of human serum alkaline phosphatase: a cross-sectional study. Metabolism. 1995; 44(9):1190–1193.
Article35. Kaviarasan S, Naik G, Gangabhagirathi R, Anuradha C, Priyadarsini K. In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum graecum) seeds. Food Chem. 2007; 103(1):31–37.36. Genet S, Kale RK, Baquer NZ. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: Effect of vanadate and fenugreek (Trigonella foenum graecum). Mol Cell Biochem. 2002; 236(1-2):7–12.37. Kaviarasan S, Anuradha CV. Fenugreek (Trigonella foenum graecum) seed polyphenols protect liver from alcohol toxicity: a role on hepatic detoxification system and apoptosis. Pharmazie. 2007; 62(4):299–304.38. Kaviarasan S, Sundarapandiyan R, Anuradha CV. Protective action of fenugreek (Trigonella foenum graecum) seed polyphenols against alcohol-induced protein and lipid damage in rat liver. Cell Biol Toxicol. 2008; 24(5):391–400.
Article39. Kaviarasan S, Ramamurty N, Gunasekaran P, Varalakshmi E, Anuradha CV. Fenugreek (Trigonella foenum graecum) seed extract prevents ethanol-induced toxicity and apoptosis in Chang liver cells. Alcohol Alcohol. 2006; 41(3):267–273.
Article40. Hannan JM, Rokeya B, Faruque O, Nahar N, Mosihuzzaman M, Azad Khan AK, et al. Effect of soluble dietary fibre fraction of Trigonella foenum graecum on glycemic, insulinemic, lipidemic and platelet aggregation status of Type 2 diabetic model rats. J Ethnopharmacol. 2003; 88(1):73–77.
Article41. Belaïd-Nouira Y, Bakhta H, Haouas Z, Flehi-Slim I, Neffati F, Najjar MF, et al. Fenugreek seeds, a hepatoprotector forage crop against chronic AlCl3 toxicity. BMC Vet Res. 2013; 9:22.
Article42. Borges LP, Brandão R, Godoi B, Nogueira CW, Zeni G. Oral administration of diphenyl diselenide protects against cadmium-induced liver damage in rats. Chem Biol Interact. 2008; 171(1):15–25.
Article43. Devi PS, Shyamala DC. Protective effect of quercetin in cisplatin-induced cell injury in the rat kidney. Ind J Pharmacol. 1999; 31(6):422–426.44. Xue W, Lei J, Li X, Zhang R. Trigonella foenum graecum seed extract protects kidney function and morphology in diabetic rats via its antioxidant activity. Nutr Res. 2011; 31(7):555–562.
Article45. Kaviarasan S, Viswanathan P, Anuradha CV. Fenugreek seed (Trigonella foenum graecum) polyphenols inhibit ethanol-induced collagen and lipid accumulation in rat liver. Cell Biol Toxicol. 2007; 23(6):373–383.
Article46. Gupta A, Gupta R, Lal B. Effect of Trigonella foenum-graecum (fenugreek) seeds on glycaemic control and insulin resistance in type 2 diabetes mellitus: a double blind placebo controlled study. J Assoc Physicians India. 2001; 49:1057–1061.47. Gupta D, Raju J, Baquer NZ. Modulation of some gluconeogenic enzyme activities in diabetic rat liver and kidney effect of anti-diabetic compounds. Indian J Exp Biol. 1999; 37:196–199.48. Raju J, Gupta D, Rao AR, Yadava PK, Baquer NZ. Trigonella foenum graecum (fenugreek) seed powder improves glucose homeostasis in alloxan diabetic rat tissues by reversing the altered glycolytic, gluconeogenic and lipogenic enzymes. Mol Cell Biochem. 2001; 224(1-2):45–51.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Trigonella foenum-graceum L. (Fenugreek) Towards Collagen Type I Alpha 1 (COL1A1) and Collagen Type III Alpha 1 (COL3A1) on Postmenopausal Woman's Fibroblast
- Serum Aluminum Level in End-Stage Renal Failure
- Aluminum Clearance by Hemodialysis in Chronic Renal Failure
- A Case Report of Acute Encephalopathy by Aluminum Intoxication with Subdural Hematoma in End Stage Renal Failure
- A Case Report of High-turnover Renal Osteodystrophy with Positive Aluminum Staining