Nutr Res Pract.
2012 Oct;6(5):389-395.
Carbohydrase inhibition and anti-cancerous and free radical scavenging properties along with DNA and protein protection ability of methanolic root extracts of Rumex crispus
- Affiliations
-
- 1Department of Medical Biotechnology, College of Biomedical Sciences, Kangwon National University, 192-1 Hyoja-dong, Chuncheon-si, Gangwon 200-701, Korea. mhwang@kangwon.ac.kr
- 2Department of Animal Biotechnology, College of Animal Life Sciences, Kangwon National University, Gangwon 200-701, Korea.
Abstract
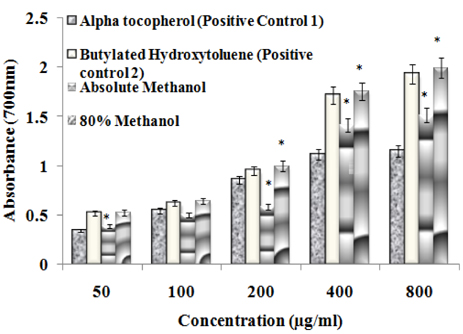
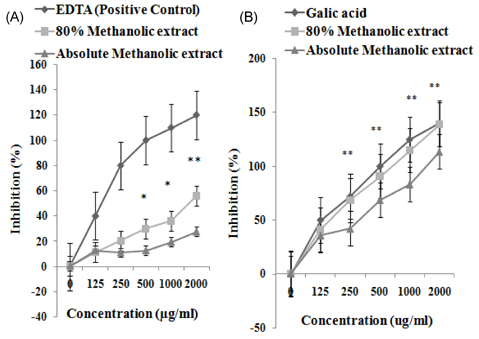
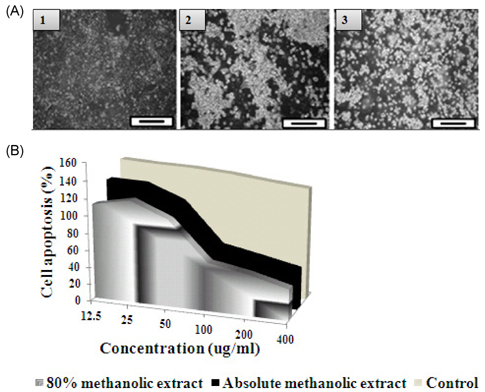
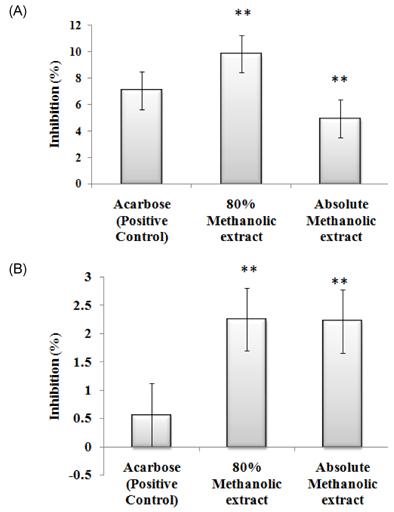
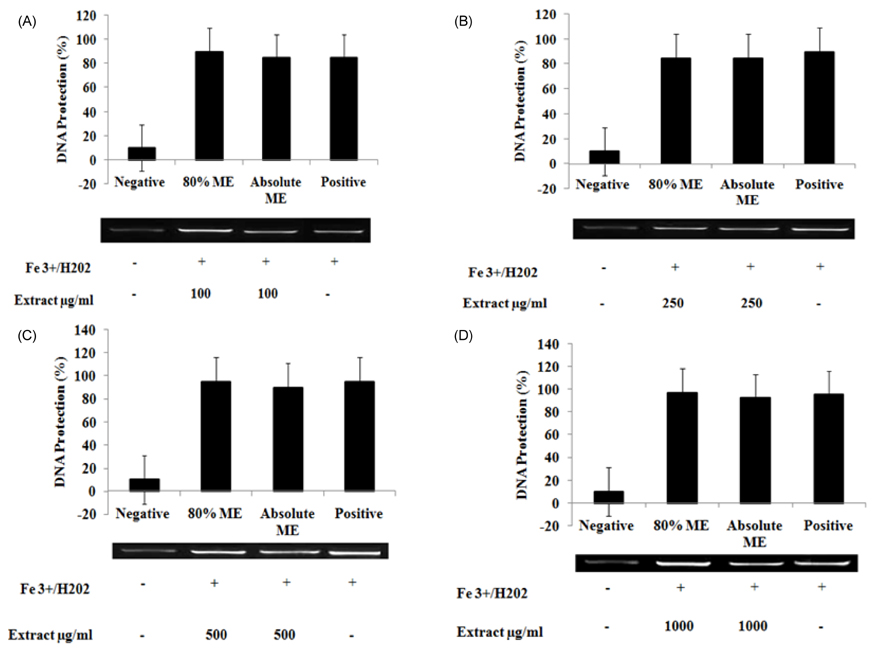
- The study elucidated carbohydrase inhibition, anti-cancerous, free radical scavenging properties and also investigated the DNA and protein protection abilities of methanolic root extract of Rumex crispus (RERC). For this purpose, pulverized roots of Rumex crispus was extracted in methanol (80% and absolute conc.) for 3 hrs for 60degrees C and filtered and evaporated with vacuum rotary evaporator. RERC showed high phenolic content (211 microg/GAE equivalent) and strong 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging (IC50 = 42.86 (absolute methanol) and 36.91 microg/mL (80% methanolic extract)) and reduced power ability. Furthermore, RERC exhibited significant protective ability in H2O2/Fe3+/ascorbic acid-induced protein or DNA damage and percentage inhibition of the HT-29 cell growth rate following 80% methanolic RERC exposure at 400 microg/mL was observed to be highest (10.2% +/- 1.03). Moreover, methanolic RERC inhibited alpha-glucosidase and amylase effectively and significantly (P < 0.05). Conclusively, RERC could be considered as potent carbohydrase inhibitor, anti-cancerous and anti-oxidant.
Keyword
MeSH Terms
Figure
Reference
-
1. Hazra B, Sarkar R, Mandal S, Biswas S, Mandal N. Studies on antioxidant and antiradical activities of Dolichos biflorus seed extract. Afr J Biotechnol. 2009. 8:3927–3933.2. Espín JC, Soler-Rivas C, Wichers HJ. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphenyl-1-picrylhydrazyl radical. J Agric Food Chem. 2000. 48:648–656.
Article3. Slavnić Ž. Josifović M, editor. Polygonales-Polygonaceae. Flora of SR Serbia. 1972. Vol. III. Belgrade: Serbian Academy of Sciences and Arts;68–86.4. Yıldırım A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem. 2001. 49:4083–4089.
Article5. Tyler VE. The Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedies. 1993. 3rd ed. New York: Pharmaceutical Products Press, An imprint of the Haworth Press, Inc.;325–326.6. Burrill LC. Curly Dock and Broadleaf Dock, Rumex Crispus L. and Rumex Obtusifolius L. 1992. Oregon State University Extension Service, Washington State University Extension Service, University of Idaho Extension Service;Available from: http://ir.library.oregonstate.edu/xmlui/bitstream/handle/1957/16791/pnw398.pdf.7. Rutledge CR, McLendon T. An Assessment of Exotic Plant Species of Rocky Mountain National Park: Summary Information for Remaining Exotic Plant Species. 2002. Colorado: Department of Rangeland Ecosystem Science, Colorado State University;97. United States Geological Survey, Northern Prairie Wildlife Research Center Home Page. Available from: http://www.npwrc.usgs.gov/resource/plants/explant/index.htm.8. Hu W, Shen W, Wang MH. Free radical scavenging activity and protective ability of methanolic extract from Duchesnea indica against protein oxidation and DNA damage. J Food Sci Nutr. 2009. 14:277–282.
Article9. Hu W, Heo SI, Wang MH. Antioxidant and anti-inflammatory activity of Kalopanax pictus leaf. J Korean Soc Appl Biol Chem. 2009. 52:360–366.10. Ghasemi K, Ghasemi Y, Ebrahimzadeh MA. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak J Pharm Sci. 2009. 22:277–281.11. Que F, Mao L, Zhu C, Xie G. Antioxidant properties of Chinese yellow wine, its concentrate and volatiles. Lebenson Wiss Technol. 2006. 39:111–117.
Article12. Singh N, Rajini PS. Free radical scavenging activity of an aqueous extract of potato peel. Food Chem. 2004. 85:611–616.
Article13. McDougall GJ, Shpiro F, Dobson P, Smith P, Blake A, Stewart D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J Agric Food Chem. 2005. 53:2760–2766.
Article14. Ali H, Houghton PJ, Soumyanath A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol. 2006. 107:449–455.
Article15. Kızıl G, Kızıl M, Ceken B. Protective ability of ethanol extracts of Hypericum scabroides Robson & Poulter and Hypericum triquetrifolium Turra against protein oxidation and DNA damage. Food Sci Biotechnol. 2009. 18:130–136.16. Qian ZJ, Jung WK, Byun HG, Kim SK. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster, Crassostrea gigas against free radical induced DNA damage. Bioresour Technol. 2008. 99:3365–3371.
Article17. Frankel EN. Natural and biological antioxidants in food and biological systems, their mechanism of action, applications and implications. Lipid Technol. 1995. 7:77–80.18. Kızıl G, Kızıl M, Ceken B, Yavuz M, Demir H. Protective ability of ethanol extracts of Hypericum scabrum L. and Hypericum retusum aucher against the protein oxidation and DNA damage. Int J Food Prop. 2011. 14:926–940.
Article19. Loft S, Fischer-Nielsen A, Jeding IB, Vistisen K, Poulsen HE. 8-Hydroxydeoxyguanosine as a urinary biomarker of oxidative DNA damage. J Toxicol Environ Health. 1993. 40:391–404.
Article20. Je JY, Ahn CB, Oh MJ, Kang SY. Antioxidant activity of a red seaweed Polysiphonia morrowii extract. Food Sci Biotechnol. 2009. 18:124–129.21. Yamagishi S, Nakamura K, Takeuchi M. Inhibition of postprandial hyperglycemia by acarbose is a promising therapeutic strategy for the treatment of patients with the metabolic syndrome. Med Hypotheses. 2005. 65:152–154.
Article22. Ferrari CK, Torres EA. Biochemical pharmacology of functional foods and prevention of chronic diseases of aging. Biomed Pharmacother. 2003. 57:251–260.
Article23. Wang J, Yuan X, Jin Z, Tian Y, Song H. Free radical and reactive oxygen species scavenging activities of peanut skins extract. Food Chem. 2007. 104:242–250.
Article24. Je JY, Kim SK. Reactive oxygen species scavenging activity of aminoderivatized chitosan with different degree of deacetylation. Bioorg Med Chem. 2006. 14:5989–5994.
Article25. Ayed-Boussema I, Bouaziz C, Rjiba K, Valenti K, Laporte F, Bacha H, Hassen W. The mycotoxin Zearalenone induces apoptosis in human hepatocytes (HepG2) via p53-dependent mitochondrial signaling pathway. Toxicol In Vitro. 2008. 22:1671–1680.
Article26. Chiu SM, Xue LY, Usuda J, Azizuddin K, Oleinick NL. Bax is essential for mitochondrion-mediated apoptosis but not for cell death caused by photodynamic therapy. Br J Cancer. 2003. 89:1590–1597.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Free radical scavenging and antioxidant enzyme fortifying activities of extracts from Smilax china root
- Effects of Antioxidative, DPPH Radical Scavenging Activity and Antithrombogenic by the Extract of Sancho ( Zanthoxylum Schinifolium)
- Antioxidant and antidiabetic activities of extracts from Cirsium japonicum roots
- A Study of Antifungal Activity with Rumex japonicus Houttuyn
- Anti-inflammatory and Anti-oxidant Effects of Sophora flavescens Root Extraction in Lipopolysaccharide-activated Raw 264.7 Cells