Nutr Res Pract.
2009 Sep;3(3):180-184.
beta-Glucan enhanced apoptosis in human colon cancer cells SNU-C4
- Affiliations
-
- 1Department of Food and Nutrition, Dongduk Women's University, 23-1 Wolgok-dong Sungbuk-gu, Seoul 136-714, Korea.
- 2Department of Fermented Food Science, Seoul university of Venture & Information, 37-18 Samsung-dong, Kangnam-gu, Seoul 135-090, Korea. sakang@suv.ac.kr
- 3Department of Agrofood Resources, National Academy of Agricultural Science, RDA, 160 Nokjiro, Gwonseon-gu, Suwon, Gyeonggi 441-853, Korea.
- 4Department of Food and Nutrition, Kangwon National University, Joongang-ro, Samcheok, Gangwon 245-711, Korea.
- 5Molecular Bioprocess Research Center, Korea Research Institute of Bioscience and Biotechnology, 1404 Jeongeup, Jeonbuk 580-185, Korea.
Abstract
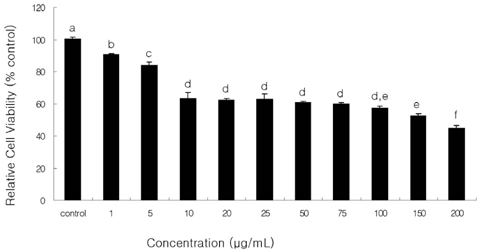
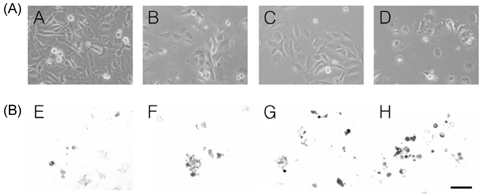
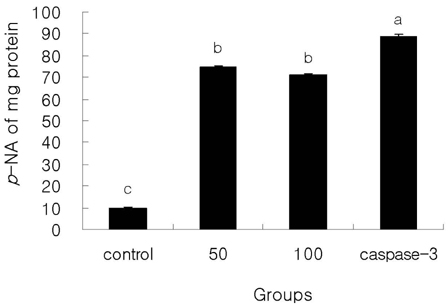
- The apoptotic effect of bacteria-derived beta-glucan was investigated in human colon cancer cells SNU-C4 using terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) assay, reverse transcription-polymerase chain reaction (RT-PCR) expressions of Bcl-2, Bax, and Caspase-3 genes, and assay of caspase-3 enzyme activity. beta-Glucan of 10, 50, and 100 microg/mL decreased cell viability in a dose-dependent manner with typical apoptotic characteristics, such as morphological changes of chromatin condensation and apoptotic body formation from TUNEL assay. In addition, beta-glucan (100 microgram/mL) decreased the expression of Bcl-2 by 0.6 times, whereas the expression of Bax and Caspase-3 were increased by 3.1 and 2.3 times, respectively, compared to untreated control group. Furthermore, the caspase-3 activity in the beta-glucan-treated group was significantly increased compared to those in control group (P < 0.05). Bacterial derived beta-glucan could be used as an effective compound inducing apoptosis in human colon cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Behall KM, Scholfield DJ, Hallfrisch J. Effect of β-glucan level in oat fiber extracts on blood lipids in men and women. J Am Coll Nutr. 1997. 16:46–51.
Article2. Cohen JJ. Apoptosis. Immunol Today. 1993. 14:126–130.
Article3. Davidson MH, Dugan LD, Burns JH, Bova J, Story K, Drennan KB. The ypocholesterolemic effects of β-glucan in oatmeal and oat bran: a dose-controlled study. JAMA. 1991. 265:1833–1839.
Article4. Harlozinska A. Progress in molecular mechanisms of tumor metastasis and angiogenesis. Anticancer Res. 2005. 25:3327–3333.5. Hong KH, Jang KH, Lee JC, Kim SH, Kim MK, Lee IY, Kim SM, Lim YH, Kang SA. Bacterial β-glucan exhibits potent hypoglycemic activity via decrease of serum lipids and adiposity, and increase of UCP mRNA expression. J Microbiol Biotechnol. 2005. 15:823–830.6. Kobayashi H, Yoshida R, Kanada Y, Fukuda Y, Yagyu T, Inagaki K, Kondo T, Kurita N, Suzuki M, Kanayama N, Terao T. Suppressing effects of daily oral supplementation of β-glucan extracted from Agaricus blazei Murill on spontaneous and peritoneal disseminated metastasis in mouse model. J Cancer Res Clin Oncol. 2005. 131:527–538.
Article7. McIntosh GH, Whyte J, McArthur R, Nestel PJ. Barley and wheat foods: influence on plasma cholesterol concentrations in hypercholesterolemic men. Am J Clin Nutr. 1991. 53:1205–1209.
Article8. Moudy AM, Handran SD, Goldberg MP, Ruffin N, Karl I, Kranz-Eble P, DeVivo DC, Rothman SM. Abnormal calcium homeostasis and mitochondrial polarization in a human encephalomyopathy. Proc Natl Acad Sci U S A. 1995. 92:729–733.
Article9. Nameda S, Miura NN, Adachi Y, Ohno N. Antibiotics protect against septic shock in mice administered β-glucan and indomethacin. Microbiol Immunol. 2007. 51:851–859.
Article10. Newman RK, Lewis SE, Newman CW, Bioik RJ, Ramage RT. Hypocholesterolemic effects of barley food on healthy men. Nutr Rep Int. 1989. 34:749–760.11. Nicolosi R, Bell SJ, Bistrian BR, Greenberg I, Forse RA, Blackburn GL. Plasma lipid changes after supplementation with β-glucan fiber from yeast. Am J Clin Nutr. 1999. 70:208–212.
Article12. Novak M, Vetvicka V. β-glucans, history, and the present: immunomodulatory aspects and mechanisms of action. J Immunotoxicol. 2008. 5:47–57.
Article13. Ohno N, Egawa Y, Hashimoto T, Adachi Y, Yadomae T. Effect of β-glucans on the nitric oxide synthesis by peritoneal macrophage in mice. Biol Pharm Bull. 1996. 19:608–612.
Article14. Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993. 74:609–619.
Article15. Park HJ, Kim MJ, Ha E, Chung JH. Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine. 2008. 15:147–151.
Article16. Qiao L, Hanif R, Sphicas E, Shiff SJ, Rigas B. Effect of aspirin on induction of apoptosis in HT-29 human colon adenocarcinoma cells. Biochem Pharmacol. 1998. 55:53–64.
Article17. Siripong P, Hahnvajanawong C, Yahuafai J, Piyaviriyakul S, Kanokmedhakul K, Kongkathip N, Ruchirawat S, Oku N. Induction of Apoptosis by Rhinacanthone isolated from Rhinacanthus nasutus roots in human cervical carcinoma cells. Biol Pharm Bull. 2009. 32:1251–1260.
Article18. Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD Jr, Chen LB. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A. 1991. 88:3671–3675.
Article19. Volman JJ, Ramakers JD, Plat J. Dietary modulation of immune function by β-glucans. Physiol Behav. 2008. 94:276–284.
Article20. Yamamoto K, Kimura T, Sugitachi A, Matsuura N. Anti-angiogenic and anti-metastatic effects of β-1,3-D-glucan purified from Hanabiratake, Sparassis crispa. Biol Pharm Bull. 2009. 32:259–263.
Article21. Yang ZG, Chen AQ, Liu B. Antiproliferation and apoptosis induced by evodiamine in human colorectal carcinoma cells (COLO-205). Chem Biodivers. 2009. 6:924–933.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characteristics of [ 18F ] fluorodeoxyglucose Uptake in Human Colon Cancer Cells
- Immunostimulatory Effects of beta-glucan Purified from Paenibacillus polymyxa JB115 on Mouse Splenocytes
- Determination of Glucan Contents in the Fruiting Bodies and Mycelia of Lentinula edodes Cultivars
- Maturation of bone marrow-derived dendritic cells by a novel beta-glucan purified from Paenibacillus polymyxa JB115
- Naringenin-Mediated ATF3 Expression Contributes to Apoptosis in Human Colon Cancer