Nutr Res Pract.
2008 Sep;2(3):143-151.
Comparison of anti-oxidant activities of seventy herbs that have been used in Korean traditional medicine
- Affiliations
-
- 1Major in Food and Nutrition, College of Human Ecology, Sookmyung Women's University, 52 Hyochangwon-gil, Yongsan-gu, Seoul 140-742, Korea. hskim@sookmyung.ac.kr
- 2Department of Pharmacology, Seoul National University School of Medicine, 28 Yeongeon-dong, Jongro-gu, Seoul 110-799, Korea. mhchung@snu.ac.kr
- 3Department of Family Medicine, Hallym University Sacred Heart Hospital, 896 Pyeonchon-dong, Dongan-gu, Anyang 431-070, Korea.
Abstract
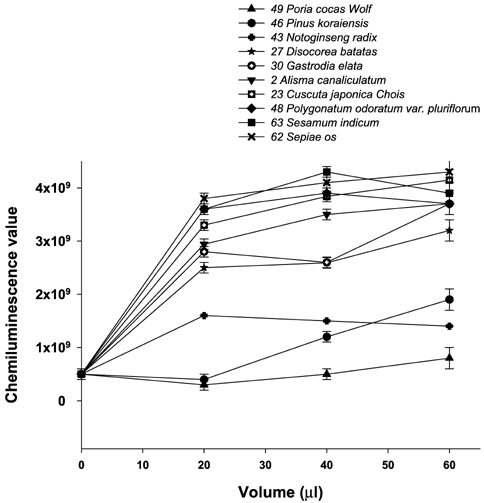
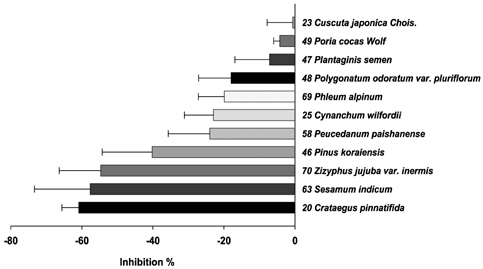
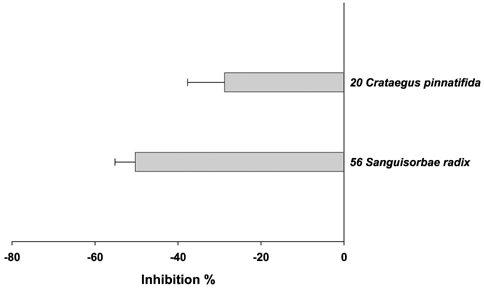
- Many herbs have been used as therapeutics in Korean traditional medicine. In view of their clinical indications, anti-oxidant activity may contribute to their pharmacological effects. However, anti-oxidant information on these plants has not been available. In this study, seventy herbs which have been used in Korean traditional medicine were selected and screened for anti-oxidant activity using their water extracts. The anti-oxidant activity was assessed by their ability to inhibit three oxidation reactions; luminol/Fenton reagent, 2, 7-dichlorodihydrofluorescein (DCHF)/Fenton reagent and DCHF/peroxynitrite. In each assay, 70 herbs were divided into two groups; anti-oxidant group which inhibited the respective oxidation reaction and was majority (about 60 herbs), and pro-oxidant group which enhanced the oxidation reaction but was minority (more or less 10 herbs). When the herbs were listed in the order of their anti-oxidant strength, the orders obtained from each assay were found to be quite similar. The upper top rankers (more or less 10 herbs) in each assay showed strong activity compared to the others. The uppermost rankers in each assay were Rubus coreanus Miquel/ Rubus schizostylus, Schisandra chinensis Baillon/ Schizandra chinensis and Terminalia chebula Retzius/ Terminalia chebula. Of the pro-oxidant herbs, about 4-5 herbs were strongly pro-oxidant, which enhanced the control oxidation reactions to 150-300%. But the meaning of this observation is not known since few of them in one assay were also anti-oxidant in other assays. The results obtained in the present study may serve as information for understanding pharmacological effects of these herbs and developing new drugs from them.
Keyword
MeSH Terms
Figure
Reference
-
1. Bagchi D, Bagdhi M, Hassoun SJ, Stochs SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995. 104:129–140.
Article2. Bent S, Ko R. Commonly used herbal medicines in the United States: a review. Am J Med. 2004. 116:478–485.
Article3. Bushman BS, Phillips B, Isbell T, Ou B, Crane JM, Knapp SJ. Chemical composition of craneberry (Rubus spp.) seeds and oils and their antioxidant potential. J Agric Food Chem. 2004. 52:7982–7987.
Article4. Catapano AL, Maggi FM, Tragni E. Low density lipoprotein oxidation, antioxidants, and atherosclerosis. Curr Opin Cardiol. 2000. 15:355–363.
Article5. Chang IS. Treatise on Asian herbal Medicines. 2003. Vol. 1. Seoul, Republic of Korea: Natural Products Research Institute, Seoul National University publishing department;2.6. Eder K, Flader D, Hirche F, Brandsch C. Excess dietary vitamin E lowers the activities of antioxidative enzymes in erythrocytes of rats fed salmon oil. J Nutr. 2002. 132:3400–3404.
Article7. Erdemoglu N, Kupeli E, Yesilada E. Anti-inflammatory and antinociceptive activity assessment of plants used as remedy in Turkish folk medicine. J Ethnopharmacol. 2003. 89:123–129.
Article8. Gandhi NM, Nair CK. Radiation protection by Terminalia chebula: some mechanistic aspects. Mol Cell Biochem. 2005. 277:43–48.
Article9. Halliwell , Gutteridge . Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. 1984. 1:1396–1397.
Article10. Hausladen A, Stamler JS. Nitrosative stress. Meth Enzymol. 1999. 300:389–395.
Article11. Jakubowski W, Bartoz G. 2, 7-dichlorofluorescin oxidation and reactive oxygen species: What does it measure? Cell Biol Int. 2000. 24:757–760.
Article12. Koteswara RN, Nammi S. Antidiabetic and renoprotective effects of the chloroform extract of Terminalia chebula Retz. seeds in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2006. 6:17–29.
Article13. 1Lee S, Suh I, Kim S. Protective effects of the green tea polyphenol (-)-epigallocatechin gallate against hippocampal neuronal damage after transient global ischemia in gerbils. Neurosci Lett. 2000a. 287:191–194.
Article14. 2Lee YM, Kim H, Hong EK, Kang BH, Kim SJ. Water extract of 1:1 mixture of Phellodendron cortex and Aralia cortex has inhibitory effects on oxidative stress in kidney of diabetic rats. J Ethnopharmacol. 2000b. 73:429–436.
Article15. Libby P. Inflammation in atherosclerosis. Nature. 2002. 420:868–874.
Article16. Liu RH. Protective role of phytochemicals in whole foods: implications for chronic disease prevention. Appl Biotechnol Food Sci Policy. 2003. 1:39–46.17. Monika B, Anurag P, Dhan P. Phenolic contents and antioxidant activity of some food and medicinal plants. Int J Food Sci Nutr. 2005. 56:287–291.
Article18. Naik GH, Priyadarsini KI, Bhagirathi RG, Mishra B, Mohan H. In vitro antioxidant studies and free radical reactions of triphala, an ayurvedic formulation and its constituents. Phytother Res. 2005. 19:582–586.
Article19. Nakatani N. Phenolic antioxidants from herbs and spices. Biofactors. 2000. 13:141–146.
Article20. Nam JH, Jung HJ, Choi J, Lee KT, Park HJ. The Anti-gastropathic Anti-rheumatic Effect of Niga-ichigoside F(1) and 23-Hydroxytormentic Acid Isolated from the Unripe Fruits of Rubus coreanus in a Rat Model. Biol Pharm Bull. 2006. 29:967–970.
Article21. Rani P, Khullar N. Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistant Salmonella typhi. Phytother Res. 2004. 18:670–673.
Article22. Siriwoharn T, Wrolstad RE, Finn CE, Pereira CB. Influence of cultivar, maturity, and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties. J Agric Food Chem. 2004. 52:8021–8030.
Article23. Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999. 283:1482–1488.
Article24. Zanon FS, Ceriatti M, Rovera LJ, Sabini BA, Ramos T. Search for antiviral activity of certain medicinal plants from Cordoba, Argentina. Rev Latinoam Microbiol. 1999. 41:59–62.25. Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001. 49:5165–5170.
Article26. Zhu H, Bannenberg G.L, Moldeus P, Shertzer HG. Oxidation pathways for the intracellular probe 2, 7-dichlorofluorescein. Arch Toxicol. 1994. 68:582–587.
Article27. Zhu YP. Chinese Materia Medica chemistry, pharmacology and applications. 1998. Netherlands: Harwood academic publishers, Inc;235–245.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Revisit to the Safety of Medicinal Herb
- Screening of Antiviral Activities of Korean Medicinal Herbs and Traditional Prescriptions Against Herpes Simplex Virus Type-1
- Alteration of hepatic anti-oxidant systems by 4-nonylphenol, a metabolite of alkylphenol polyethoxylate detergents, in Far Eastern catfish Silurus asotus
- Medicinal Herbs can Cause Cardiovascular Side Effects
- Medicinal Herbs and Toxic Hepatitis