Lab Anim Res.
2010 Sep;26(3):241-247. 10.5625/lar.2010.26.3.241.
Evaluation on Efficacy and Safety of Tribromoethanol and Tribromoethanol plus alpha2-Adrenergic Agonists in Different Mouse Strains
- Affiliations
-
- 1Department of Laboratory Animal Medicine, College of Veterinary Medicine, Konkuk University, Seoul, Korea. labvet@konkuk.ac.kr
- KMID: 2312073
- DOI: http://doi.org/10.5625/lar.2010.26.3.241
Abstract
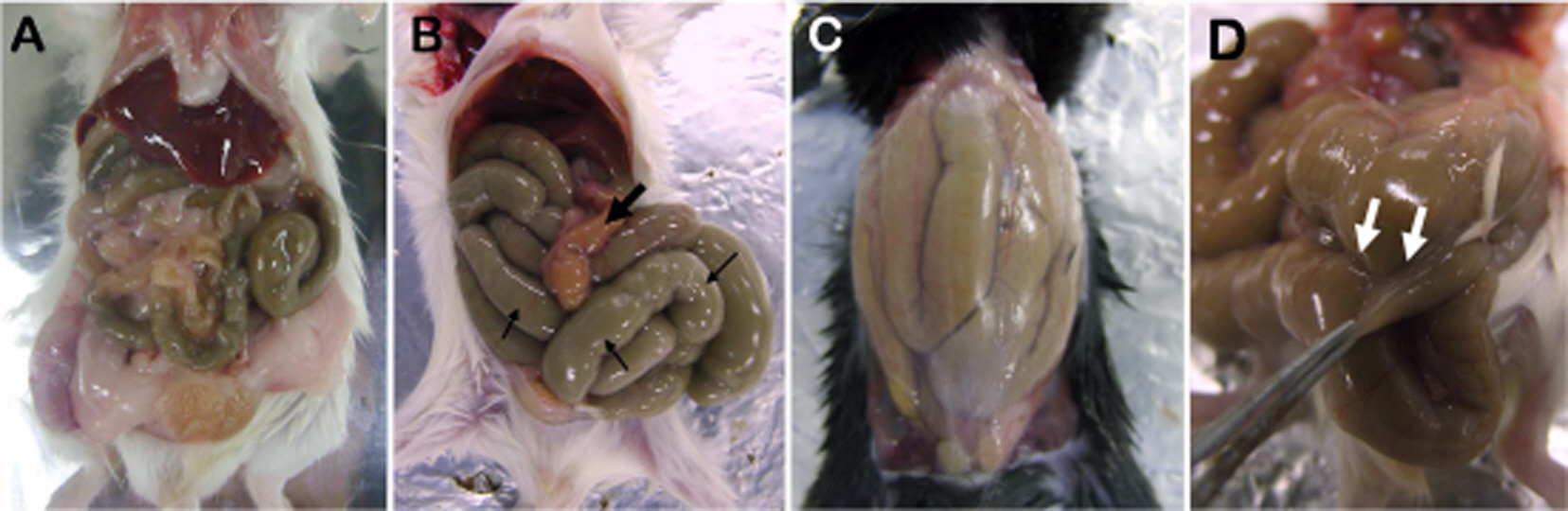
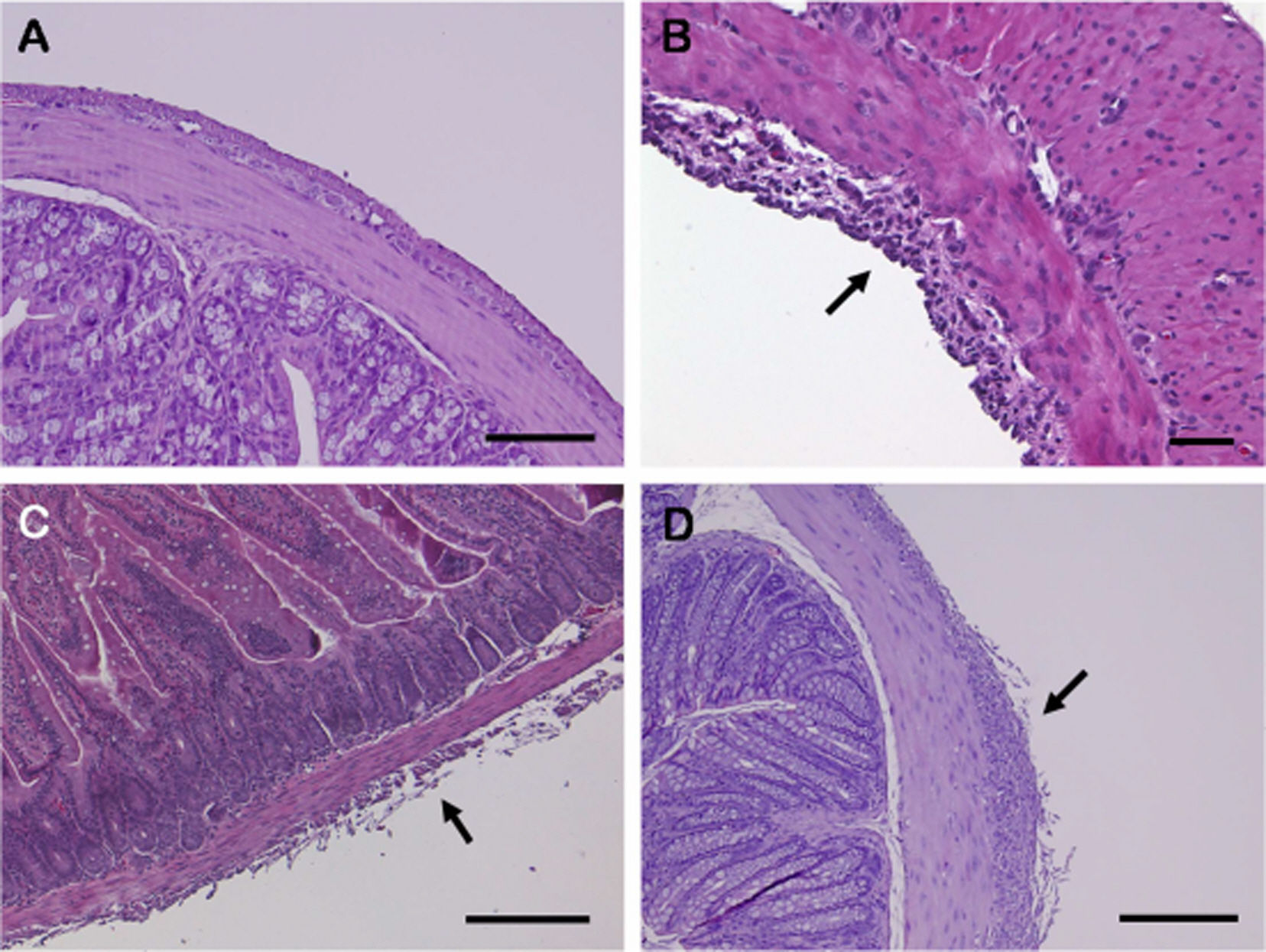
- The present study was carried out to provide a guideline for injecting tribromoethanol (TBE) as the main anesthetic agent, while adjusting the doses of xylazine (X) and medetomidine (M) according to different strains of mice (male ICR, C57BL/6, and BALB/c). Seven intraperitoneal injection anesthesia protocols using TBE and mixtures of TBE and alpha2-adrenergic agonists (TBE/X and TBE/M) were compared in terms of their efficacy and safety (anesthetic duration, death rate, and the development of pathological lesions of abdominal organs). All animals that were injected with a low dose of TBE (200 mg/kg) displayed clear signs of light anesthesia with a strong pedal withdrawal reflex. Despite the good anesthetic effect, a high dose of TBE (400 mg/kg) was not a suitable anesthetic for major surgery in all mouse strains because of the risk of pathologic changes in the abdominal organs, such as retention of the digestive tract, peritonitis, and fibrinoid adhesion. TBE200/X10 and TBE200/M0.5 (TBE, 200 mg/kg; X, 10 mg/kg; M, 0.5 mg/kg) appeared to be safe and provided satisfactory anesthesia in ICR mice. Finally, there were clear differences in anesthetic efficacy among ICR, C57BL/6, and BALB/c strains. TBE/M and TBE/X did not anesthetize BALB/c mice, and it anesthetized C57BL/6 mice for a short time. When administered with TBE/X and TBE/M maintained the sedation of ICR mice. We were able to establish different regimes for each strain (TBE200/X20 for C57BL/6, TBE300/X10 and TBE200/M1 for BALB/c). Our results showed that TBE/X and TBE/M could be recommended as an anesthetic mixture, with the dose appropriately adjusted according to mouse strain.
MeSH Terms
Figure
Reference
-
Arras M.., Autenried P.., Rettich A.., Spaeni D.., Rulicke T.2001. Optimization of intraperitoneal injection anesthesia in mice: Drugs, dosages, adverse effects, and anesthesia depth. Comp. Med. 51(5):443–456.Bette M.., Schlimme S.., Mutters R.., Menendez S.., Hoffmann S.., Schulz S.2004. Influence of different anaesthetics on proinflammatory cytokine expression in rat spleen. Lab. Anim. 38(3):272–279.Buitrago S.., Martin T.E.., Tetens-Woodring J.., Belicha-Villanueva A.., Wilding G.E.2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J. Am. Assoc. Lab. Anim. Sci. 47(1):11–17.Coria-Avila G.A.., Gavrila A.M.., Menard S.., Ismail N.., Pfaus J.G.2007. Cecum location in rats and the implications for intraperitoneal injections. Lab. Anim. (NY). 36(7):25–30.
ArticleFish R.E.., Meyer R.E.2008. Pharmacology of injectable anesthetics, sedatives, and tranquilizers. In Anesthesia and Analgesia in Laboratory Animals (Fish, R.E. ed.), 2nd ed., pp. 27-82, Academic Press, Burlington.Flecknell P.A.2005. Laboratory Animal Anaesthesia, pp. 160-171, Elsevier Academic Press, San Diego.Gardner D.J.., Davis J.A.., Weina P.J.., Theune B.1995. Comparison of tribromoethanol, ketamine/acetylpromazine, telazol/xylazine, pentobarbital, and methoxyflurane anesthesia in HSD: ICR mice. Lab. Anim. Sci. 45(2):199–204.Gopalan C.., Hegade G.M.., Bay T.N.., Brown S.R.., Talcott M.R.2005. Tribromoethanol-medetomidine combination provides a safe and reversible anesthetic effect in Sprague-Dawley rats. Contemp. Top Lab. Anim. Sci. 44(1):7–10.Green C.J.1975. Neuroleptanalgesic drug combinations in the anaesthetic management of small laboratory animals. Lab. Anim. 9(3):161–178.
ArticleHall J.C.., Heel K.A.., Papadimitriou J.M.., Platell C.1998. The pathobiology of peritonitis. Gastroenterology. 114(1):185–196.
ArticleHedenqvist P.., Hellebrekers, L.J. and Van Hoosier G.L.2003. Laboratory animal analgesia, anesthesia, and euthanasia. In Handbook of Laboratory Animal Science (Hau, J. ed.), vol. I, 2nd ed., pp. 413-457, CRC Press, Boca Raton.Hedrich H.J.., Bullock G.R.2004. The Laboratory Mouse, pp. 543-554, Elsevier Academic Press, San Diego.Kiatchoosakun S.., Kirkpatrick D.., Hoit B.D.2001. Effects of tribromoethanol anesthesia on echocardiographic assessment of left ventricular function in mice. Comp. Med. 51(1):26–29.Lieggi C.C.., Artwohl J.E.., Leszczynski J.K.., Rodriguez N.A.., Fickbohm B.L.., Fortman J.D.2005a. Efficacy and safety of stored and newly prepared tribromoethanol in ICR mice. Contemp. Top Lab. Anim. Sci. 44(1):17–22.Lieggi C.C.., Fortman J.D.., Kleps R.A.., Sethi V.., Anderson J.A.., Brown C.E.., Artwohl J.E.2005b. An evaluation of preparation methods and storage conditions of tribromoethanol. Contemp. Top Lab. Anim. Sci. 44(1):11–16.Mascia M.P.., Machu T.K.., Harris R.A.1996. Enhancement of homomeric glycine receptor function by long-chain alcohols and anaesthetics. Br. J. Pharmacol. 119(7):1331–1336.
ArticleNational Research Council. 1996. Guide for the Care and Use of Laboratory Animals, pp. 56-70, National Academy Press, Washington DC.Person B.., Wexner S.D.2006. The management of postoperative ileus. Curr. Probl. Surg. 43(1):6–65.
ArticleReid W.C.., Carmichael K.P.., Srinivas S.., Bryant J.L.1999. Pathologic changes associated with use of tribromoethanol (avertin) in the Sprague Dawley rat. Lab. Anim. Sci. 49(6):665–667.Tarin D.., Sturdee A.1972. Surgical anaesthesia of mice: Evaluation of tribromoethanol, ether, halothane and methoxyflurane and development of a reliable technique. Lab. Anim. 6(1):79–84.
ArticleThompson J.S.., Brown S.A.., Khurdayan V.., Zeynalzadedan A.., Sullivan P.G.., Scheff S.W.2002. Early effects of tribromoethanol, ketamine/xylazine, pentobarbitol, and isoflurane anesthesia on hepatic and lymphoid tissue in ICR mice. Comp. Med. 52(1):63–67.Ticku M.K.., Kulkarni S.K.1988. Molecular interactions of ethanol with GABAergic system and potential of RO15-4513 as an ethanol antagonist. Pharmacol. Biochem. Behav. 30(2):501–510.
Articlevan Goor H.., de Graaf J.S.., Grond J.., Sluiter W.J.., van der Meer J.., Bom V.J.., Bleichrodt R.P.1994. Fibrinolytic activity in the abdominal cavity of rats with faecal peritonitis. Br. J. Surg. 81(7):1046–1049.
ArticleWeiss J.., Zimmermann F.1999. Tribromoethanol (avertin) as an anaesthetic in mice. Lab. Anim. 33(2):192–193.
ArticleZeller W.., Meier G.., Burki K.., Panoussis B.1998. Adverse effects of tribromoethanol as used in the production of transgenic mice. Lab. Anim. 32(4):407–413.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Kinetics of proinflammatory cytokines after intraperitoneal injection of tribromoethanol and a tribromoethanol/xylazine combination in ICR mice
- Alpha-2 Adrenergic Agonists for the Management of Surgical Pain Patient
- The Effect of alpha2 Adrenoceptors and Imidazoline Receptors on the Mechanical Allodynia in Rats with Nerve Ligation Injury
- Clinical applications of alpha2 adrenoceptor agonist
- Comparison of the anesthetic effects of 2,2,2-tribromoethanol on ICR mice derived from three different sources