Lab Anim Res.
2010 Jun;26(2):197-201. 10.5625/lar.2010.26.2.197.
Expression Patterns of Superoxide Dismutase Genes in the Stage-specific Seminiferous Tubules of Mice Excised by a Laser Capture Microdissection
- Affiliations
-
- 1College of Veterinary Medicine & Research Institute of Veterinary Medicine, Chungbuk National University, Cheongju, Korea. synam@cbu.ac.kr
- 2Laboratory of Mammalian Molecular Genetics, Department of Biochemistry, College of Science, Yonsei University, Seoul, Korea.
- KMID: 2312065
- DOI: http://doi.org/10.5625/lar.2010.26.2.197
Abstract
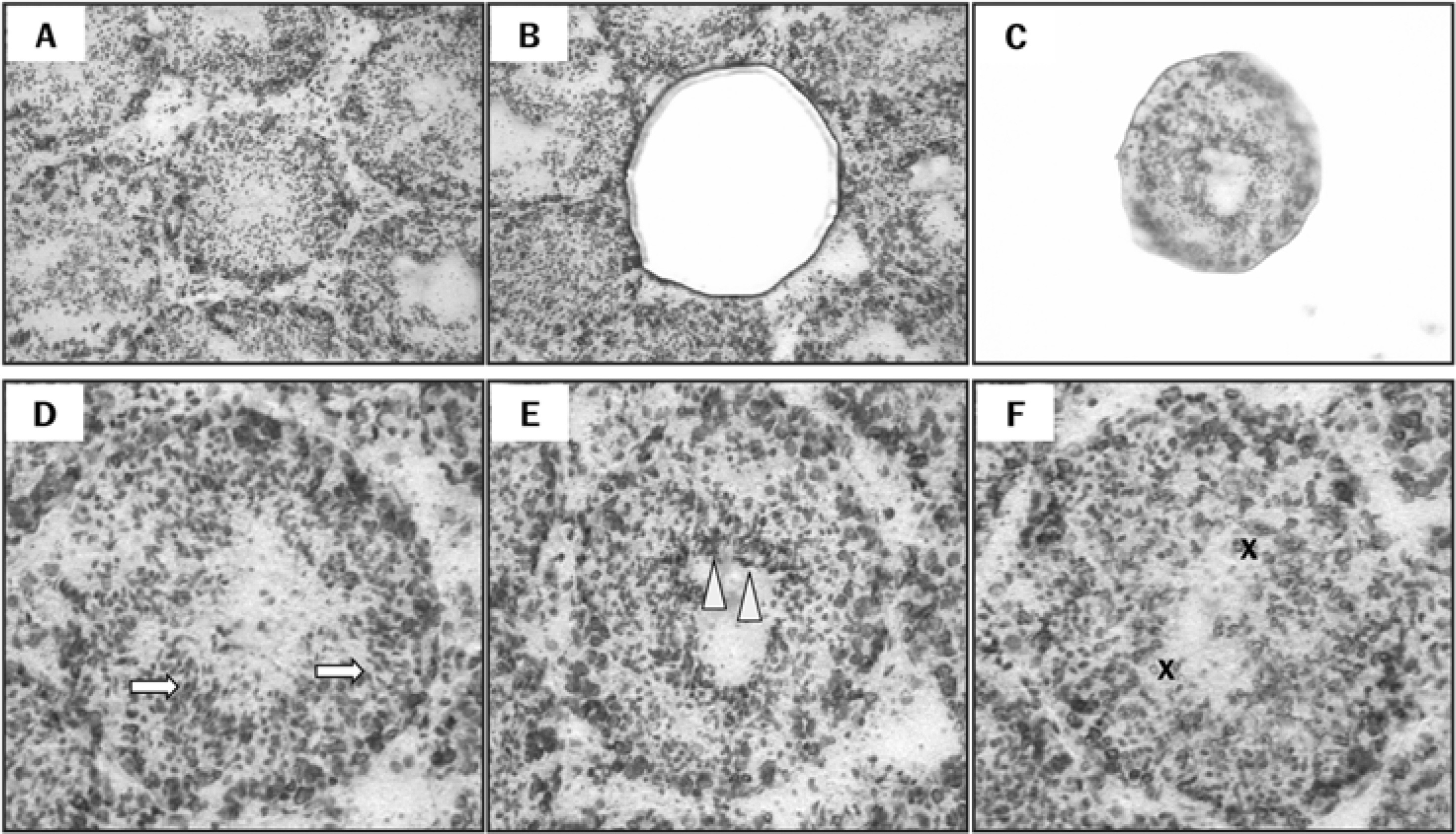
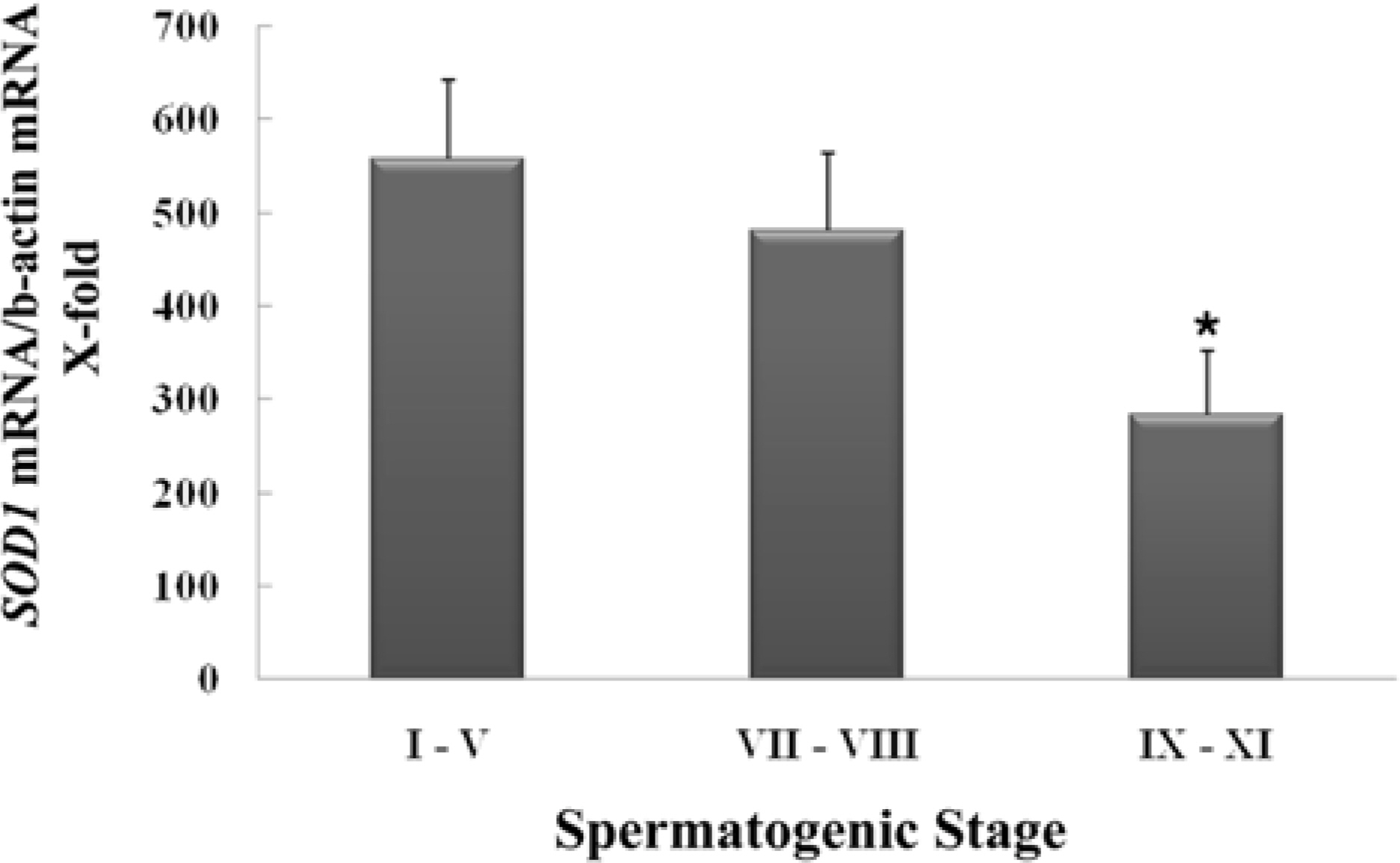
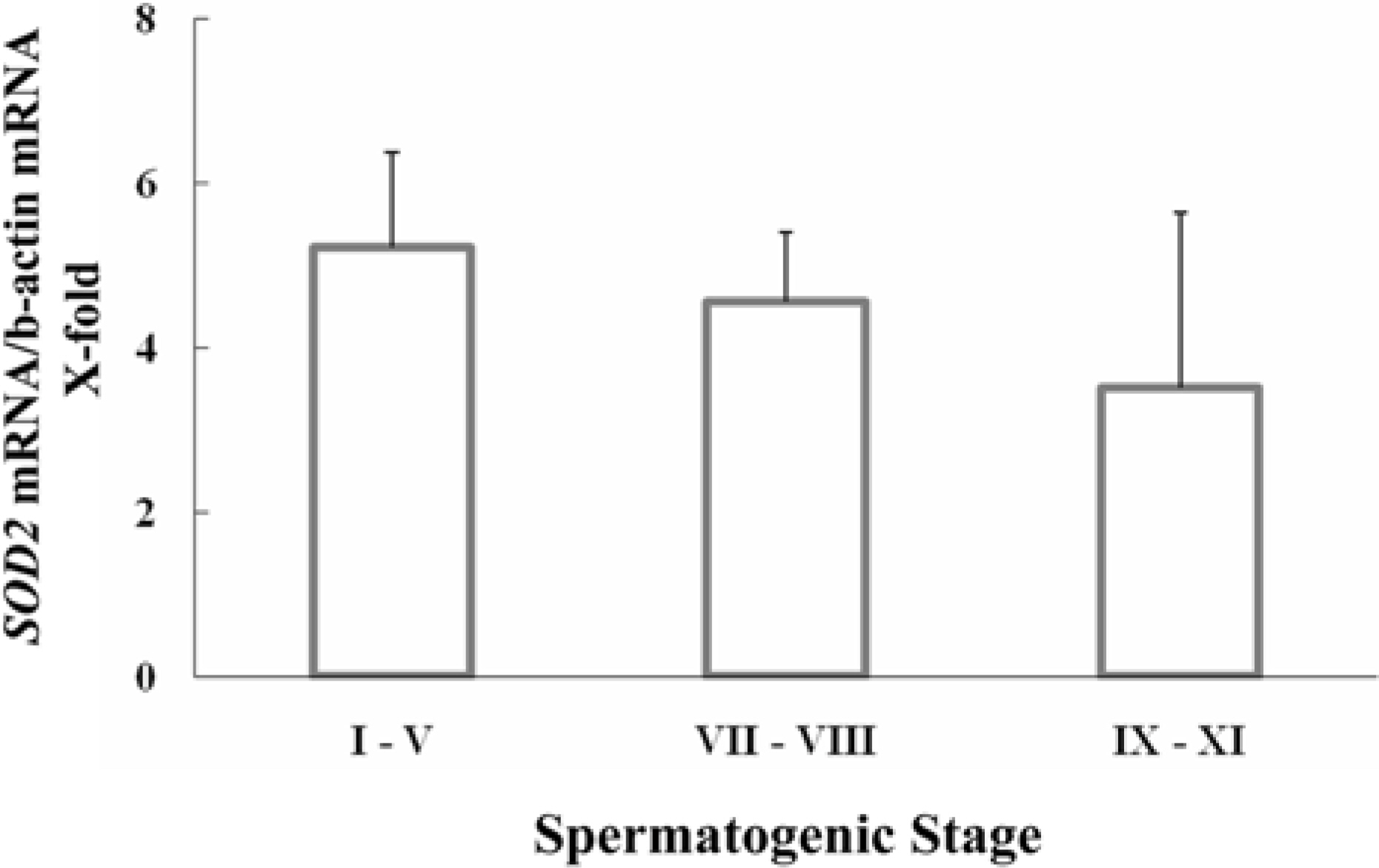
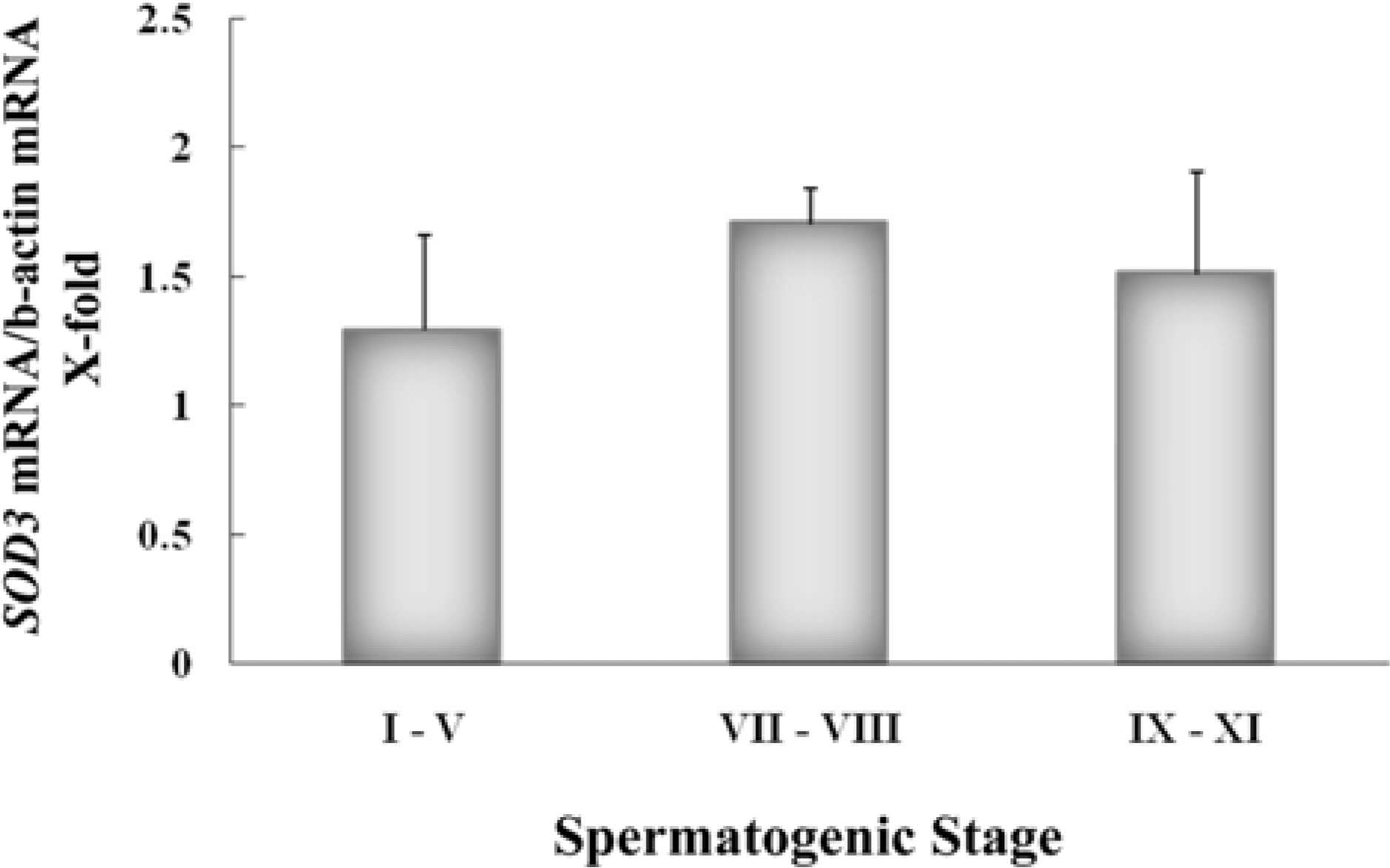
- Spermatogenesis is a particularly difficult process to study the unique multiple cellular associations within the seminiferous epithelium. Laser capture microdissection (LCM) is a recently developed technique that enables the isolation of individual cell populations from complex tissues. The superoxide dismutase (SOD) is the first and most important enzyme of antioxidant defense systems against superoxide anion. The aim of this study was to investigate the quantitative changes of SOD gene expression according to the spermatogenic cycle in mouse testes using LCM and real-time polymerase chain reaction (PCR) techniques. Frozen sections (10 micrometer) were obtained from the testes of 8-weeks-old ICR mice. LCM was used to capture all cells in cross-sectioned seminiferous tubules which were grouped into stages I-V, VII-VIII, and IX-XI. The expression level of cytoplasmic Cu, Zn-SOD (SOD1) mRNA was remarkably higher than those of mitochondrial Mn-SOD (SOD2) and extracellular Cu, Zn-SOD (SOD3) mRNAs in mouse testes. During spermatogenesis, the expressions of SOD1 and SOD2 mRNAs were highest on stages I-V, began to decrease after stage VII, and showed a lowest level on stage IX-XI. However, the expression of SOD3 mRNA was highest on stages VII-VIII. These findings suggest that the subtypes of SOD are expressed differentially in mouse testes during spermatogenesis.
MeSH Terms
Figure
Reference
-
Agarwal N.., Lippmann E.S.., Shusta E.V.2010. Identification and expression profiling of blood-brain barrier membrane proteins. J. Neurochem. 112(3):625–635.
ArticleArafa H.M.., Aly H.A.., Abd-Ellah M.F.., El-Refaey H.M.2009. Hesperidin attenuates benzo[alpha] pyrene-induced testicular toxicity in rats via regulation of oxidant/antioxidant balance. Toxicol. Ind. Health. 25(6):417–427.Bauche F.., Fouchard M.H.., Jegou B.1994. Antioxidant system in rat testicular cells. FEBS Lett. 349(3):392–396.Ben Abdallah F.., Dammak I.., Attia H.., Hentati B.., Ammar-Keskes L.2009. Lipid peroxidation and antioxidant enzyme activities in infertile men: correlation with semen parameter. J. Clin. Lab. Anal. 23(2):99–104.
ArticleChandra A.K.., Chatterjee A.., Ghosh R.., Sarkar M.2010. Vitamin E-supplementation protects chromium (VI)-induced spermatogenic and steroidogenic disorders in testicular tissues of rats. Food Chem. Toxicol. 48(3):972–979.Gu W.., Morales C.., Hecht N.B.1995. In male mouse germ cell, copper-zinc superoxide dismutase utilizes alternative promoters that produce multiple transcripts with different with different translation potential. J. Biol. Chem. 270(1):236–243.Gu W.., Hecht N.B.1996. Developmental expression of glutathione peroxidase, catalase, and manganese superoxide dismutase mRNAs during spermatogenesis in the mouse. J. Androl. 17(3):256–262.Gu W.., Hecht N.B.1997. The enzymatic activity of Cu/Zn superoxide dismutase does not fluctuate in mouse spermatogenic cells despite mRNA changes. Exp. Cell Res. 232(2):371–375.
ArticleHuang F.., Ning H.., Xin Q.Q.., Huang Y.., Wang H.., Zhang Z.H.., Xu D.X.., Ichihara G.., Ye D.Q.2009. ).Melatonin pretreatment attenuates 2-bromopropane-induced testicular toxicity in rats. Toxicology. 256(1-2):75–82.
ArticleJow W.W.., Schlegel P.N.., Cichon Z.., Phillips D.., Goldstein M.., Bardin C.W.1993. Identification and localization of copper-zinc superoxide dismutase gene expression in rat testicular development. J. Androl. 14(6):439–447.Lonnie D.R.., Ettlin R.A.., Sinha Hikim A.P.., Clegg E.D.1990. Histological and histopathological evaluation of the testis. p. 4–47. Cache River Press;USA:Mandal T.K.., Das N.S.2009. ).Effect of delta-9-tetrahydrocannabinol on altered antioxidative enzyme defense mechanisms and lipid peroxidation in mice testes. Eur. J. Pharmacol. 607(1-3):178–187.Mruk D.., Cheng C.H.., Cheng Y.H.., Mo M.Y.., Grima J.., Silvestrini B.., Lee W.M.., Cheng C.Y.1998. Rat testicular extracellular superoxide dismutase: its purification, cellular distribution, and regulation. Biol. Reprod. 59(2):298–308.Mruk D.D.., Silvestrini B.., Mo M.Y.., Cheng C.Y.2002. Antioxidant superoxide dismutase-a review: its function, regulation in the testis, and role in male fertility. Contraception. 65(4):305–311.Nonogaki T.., Noda Y.., Narimoto K.., Shiotani M.., Mori T.., Matsuda T.., Yoshida O.1992. Localization of CuZn-superoxide dismutase in the human male genital organs. Hum. Reprod. 7(1):81–85.
ArticleSluka P.., O'Donnell L.., McLachlan R.I.., Stanton P.G.2008. Application of laser-capture microdissection to analysis of gene expression in the testis. Prog. Histochem. Cytochem. 42(4):173–201.
ArticleVenkatesh S.., Deecaraman M.., Kumar R.., Shamsi M.B.., Dada R.2009. Role of reactive oxygen species in the pathogenesis of mitochondrial DNA (mtDNA) mutations in male infertility. Indian J. Med. Res. 129(2):127–137.Wang X.Z.., Liu S.S.., Sun Y.., Wu J.Y.., Zhou Y.L.., Zhang J.H.2009. Beta-cypermethrin impairs reproductive function in male mice by inducing oxidative stress. Theriogenology. 72(5):599–611.
ArticleWang S.., Wang L.., Zhu T.., Gao X.., Li J.., Wu Y.., Zhu H.2010. Improvement of tissue preparation for laser capture microdissection: application for cell type-specific miRNA expression profiling in colorectal tumors. BMC Genomics 11, 163.Zelko I.N.., Mariani T.J.., Folz R.J.2002. Superoxide dismutase multigene family: A comparion of the Cu/Zn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 33(3):337–349.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of the Gene Expression by Laser Capture Microdissection (III): Microarray Analysis of the Gene Expression at the Mouse Uterine Luminal Epithelium of the Implantation Sites during Apposition Period1
- Global Analysis of Estrogen-Regulated Genes in Mouse Uterus using cDNA Microarray and Laser Capture Microdissection
- Laser Capture Microdissection Reveals Specific Genes Related to Purkinje Cell Death in the Leaner Mice
- The change of superoxide dismutase activity in mouse skin by ultraviolet radiation
- Superoxide Dismutase Activity in Down Syndrom