J Breast Cancer.
2016 Jun;19(2):199-205. 10.4048/jbc.2016.19.2.199.
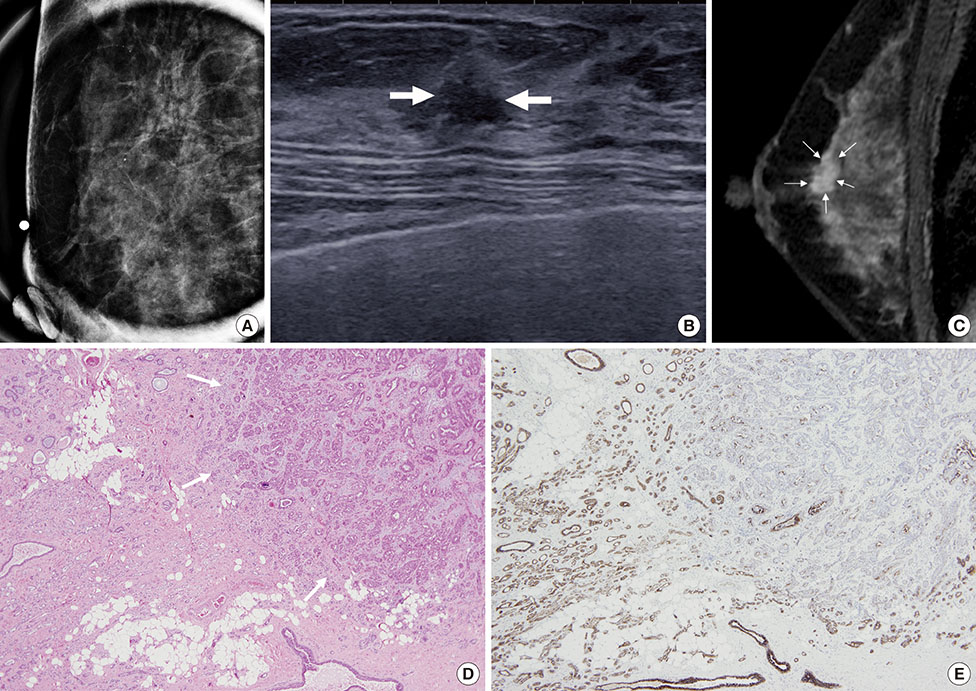
Features of Pure Lobular Carcinoma In Situ on Magnetic Resonance Imaging Associated with Immediate Re-Excision after Lumpectomy
- Affiliations
-
- 1Department of Radiology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. river7774@gmail.com
- 2Department of Radiology, Kangwon National University Graduate School, Chuncheon, Korea.
- 3Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2308972
- DOI: http://doi.org/10.4048/jbc.2016.19.2.199
Abstract
- PURPOSE
To evaluate imaging features of pure lobular carcinoma in situ (LCIS) on magnetic resonance imaging (MRI) in patients who underwent immediate re-excision after lumpectomy.
METHODS
Twenty-six patients (46.1±6.7 years) with 28 pure LCIS lesions, who underwent preoperative MRI and received curative surgery at our institution between 2005 and 2013, were included in this study. Clinicopathologic features associated with immediate re-excision were reviewed and analyzed using Fisher exact test or the Wilcoxon signed rank test.
RESULTS
Of the 28 lesions, 21.4% (6/28, six patients) were subjected to immediate re-excision due to resection margin involvement by LCIS. Nonmass lesions and moderate-to-marked background parenchymal enhancement on MRI were more frequently found in the re-excision group than in the single operation group (100% [6/6] vs. 40.9% [9/22], p=0.018; 83.3% [5/6] vs. 31.8% [7/22], p=0.057, respectively). The median lesion size discrepancy observed between magnetic resonance images and histopathology was greater in the re-excision group than in the single operation group (-0.82 vs. 0.13, p=0.018). There were no differences in the mammographic or histopathologic findings between the two groups.
CONCLUSION
Nonmass LCIS lesions or moderate-to-marked background parenchymal enhancements on MRI can result in an underestimation of the extent of the lesions and are associated with subsequent re-excision due to resection margin involvement.
MeSH Terms
Figure
Reference
-
1. Foote FW, Stewart FW. Lobular carcinoma in situ: a rare form of mammary cancer. Am J Pathol. 1941; 17:491–496.3.
Article2. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO Classification of Tumours of the Breast. Lyon: International Agency for Research on Cancer;2012.3. Hwang ES, Nyante SJ, Yi Chen Y, Moore D, DeVries S, Korkola JE, et al. Clonality of lobular carcinoma in situ and synchronous invasive lobular carcinoma. Cancer. 2004; 100:2562–2572.
Article4. Marshall LM, Hunter DJ, Connolly JL, Schnitt SJ, Byrne C, London SJ, et al. Risk of breast cancer associated with atypical hyperplasia of lobular and ductal types. Cancer Epidemiol Biomarkers Prev. 1997; 6:297–301.5. Clinical practice guidelines in oncology (NCCN Guidelines®). National Comprehensive Cancer Network. Accessed April 25th, 2015. http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.6. Morris EA, Comstock CE, Lee CH. ACR BI-RADS® magnetic resonance imaging. In : D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA, editors. ACR BI-RADS® Atlas, Breast Imaging and Reporting and Data System. 5th ed. Reston: American College of Radiology;2013.7. Buchholz TA, Somerfield MR, Griggs JJ, El-Eid S, Hammond ME, Lyman GH, et al. Margins for breast-conserving surgery with whole-breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol. 2014; 32:1502–1506.
Article8. Sadek BT, Shenouda MN, Abi Raad RF, Niemierko A, Keruakous AR, Goldberg SI, et al. Risk of local failure in breast cancer patients with lobular carcinoma in situ at the final surgical margins: is re-excision necessary? Int J Radiat Oncol Biol Phys. 2013; 87:726–730.
Article9. Ciocca RM, Li T, Freedman GM, Morrow M. Presence of lobular carcinoma in situ does not increase local recurrence in patients treated with breast-conserving therapy. Ann Surg Oncol. 2008; 15:2263–2271.
Article10. Apple SK, Matin M, Olsen EP, Moatamed NA. Significance of lobular intraepithelial neoplasia at margins of breast conservation specimens: a report of 38 cases and literature review. Diagn Pathol. 2010; 5:54.
Article11. Cao D, Polyak K, Halushka MK, Nassar H, Kouprina N, Iacobuzio-Donahue C, et al. Serial analysis of gene expression of lobular carcinoma in situ identifies down regulation of claudin 4 and overexpression of matrix metalloproteinase 9. Breast Cancer Res. 2008; 10:R91.
Article12. Gutierrez RL, DeMartini WB, Eby PR, Kurland BF, Peacock S, Lehman CD. BI-RADS lesion characteristics predict likelihood of malignancy in breast MRI for masses but not for nonmasslike enhancement. AJR Am J Roentgenol. 2009; 193:994–1000.
Article13. DeMartini WB, Liu F, Peacock S, Eby PR, Gutierrez RL, Lehman CD. Background parenchymal enhancement on breast MRI: impact on diagnostic performance. AJR Am J Roentgenol. 2012; 198:W373–W380.
Article14. Hambly NM, Liberman L, Dershaw DD, Brennan S, Morris EA. Background parenchymal enhancement on baseline screening breast MRI: impact on biopsy rate and short-interval follow-up. AJR Am J Roentgenol. 2011; 196:218–224.
Article15. Seo M, Cho N, Bae MS, Koo HR, Kim WH, Lee SH, et al. Features of undiagnosed breast cancers at screening breast MR Imaging and potential utility of computer-aided evaluation. Korean J Radiol. 2016; 17:59–68.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multi-Focal Lobular Carcinoma In Situ Arising in Benign Phyllodes Tumor: A Case Report
- Invasive Lobular Carcinoma of the Breast Associated with Mixed Lobular and Ductal Carcinoma In Situ: A Case Report
- Lobular carcinoma in situ in sclerosing adenosis
- Preoperative Magnetic Resonance Imaging FeaturesAssociated with Positive Resection Margins in Patientswith Invasive Lobular Carcinoma
- A pure mucocele-like lesion of the breast diagnosed on ultrasonography-guided core-needle biopsy: is imaging follow-up sufficient?