Anat Cell Biol.
2016 Mar;49(1):34-49. 10.5115/acb.2016.49.1.34.
Protective effect of rosemary on acrylamide motor neurotoxicity in spinal cord of rat offspring: postnatal follow-up study
- Affiliations
-
- 1Department of Anatomy and Embryology, Faculty of Medicine, Menoufia University, Shebeen El-Kom, Egypt. drhanaanooh@gmail.com
- KMID: 2308930
- DOI: http://doi.org/10.5115/acb.2016.49.1.34
Abstract
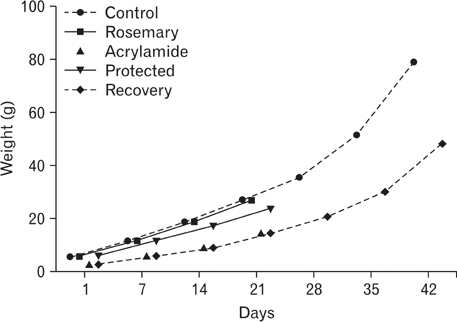
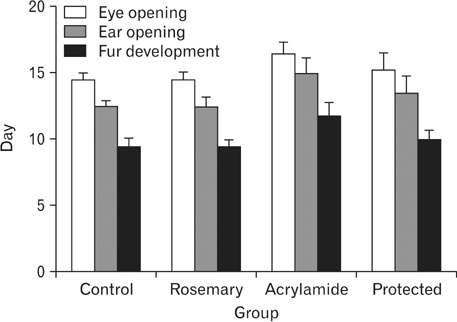
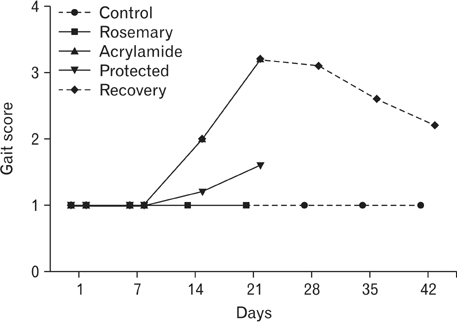
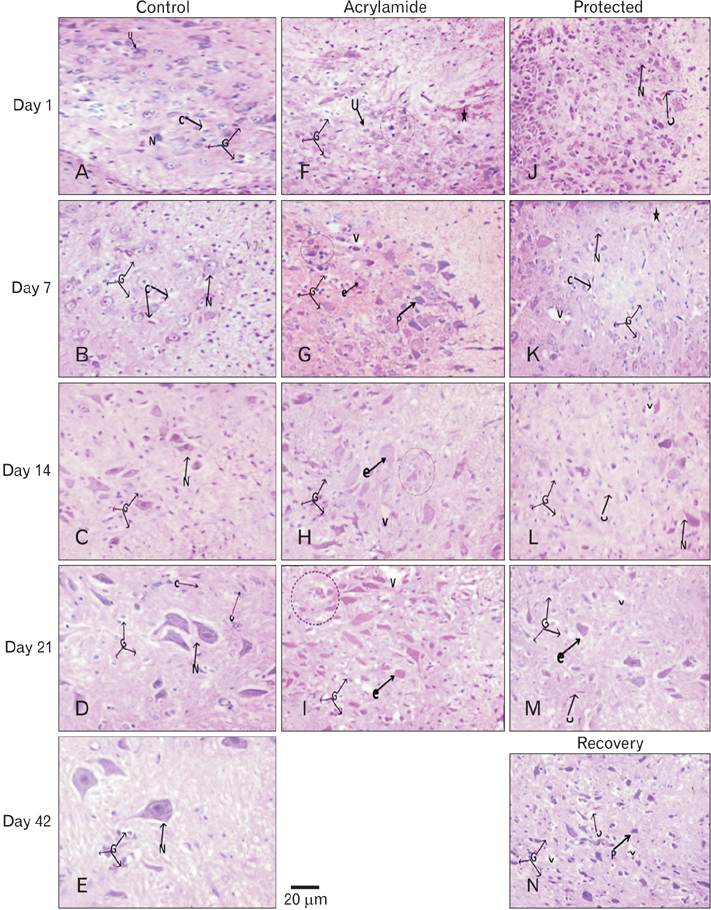
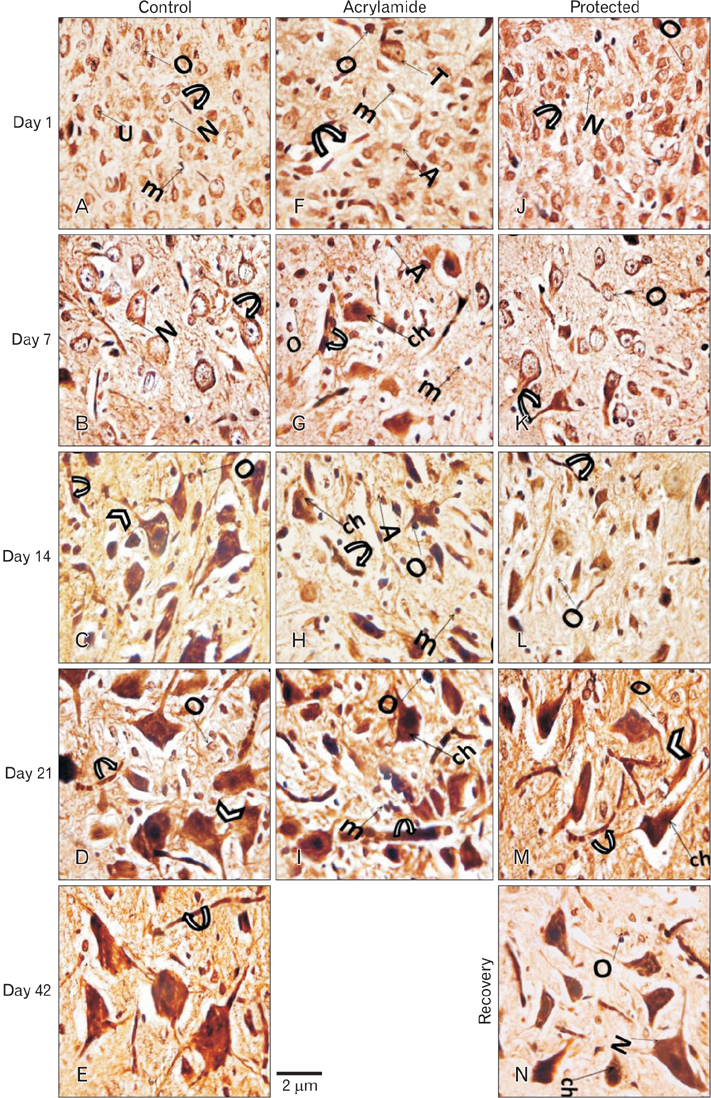
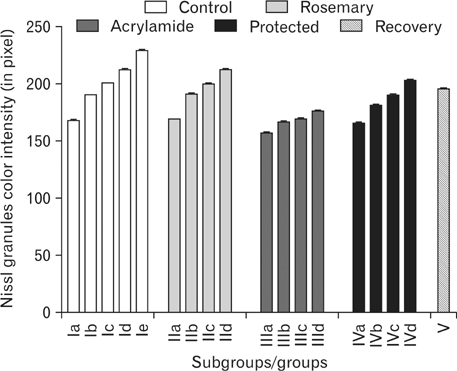
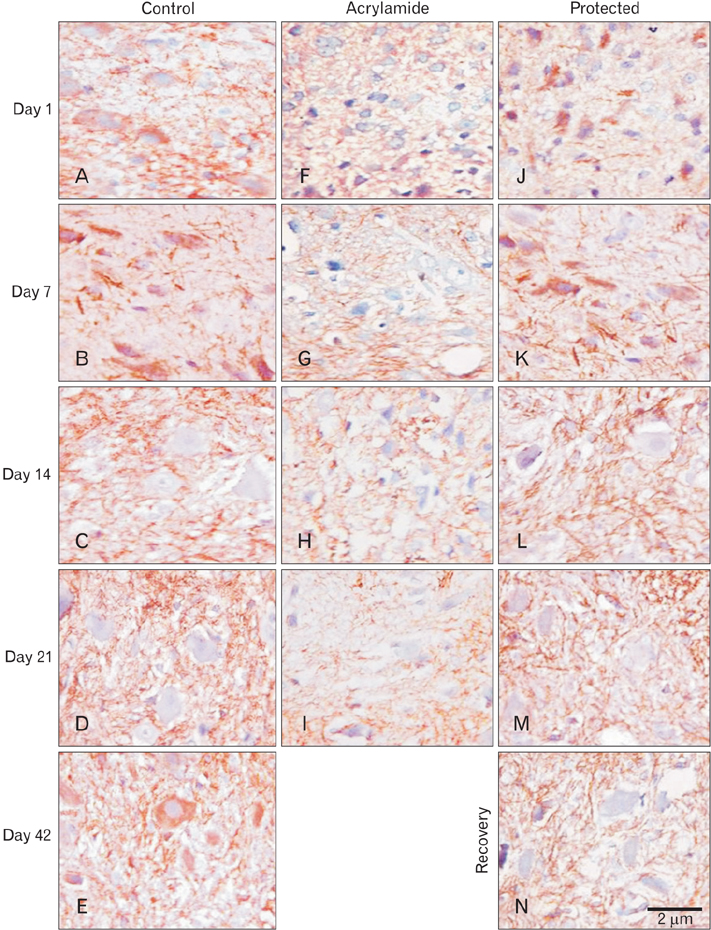
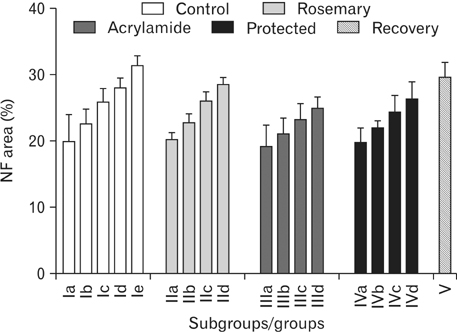
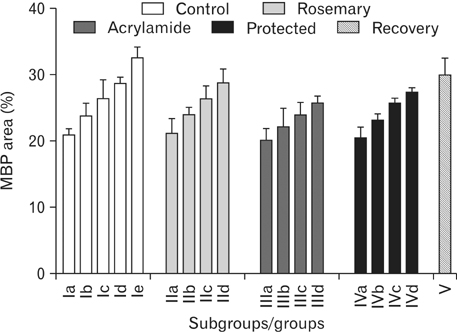
- The direct interactive effects of rosemary and acrylamide on the development of motor neurons in the spinal cord remains unknown. Our goal is to confirm the protective effects of rosemary against motor neuronal degeneration induced by acrylamide in the developing postnatal rat spinal cord using a postnatal rat model. We assigned the offspring of treated female rats into control, rosemary; acrylamide group; and recovery groups. This work depended on clinical, histopathological, morphometrically, immunohistochemical and genetic methods. In the acrylamide group, we observed oxidation, motor neuron degeneration, apoptosis, myelin degeneration, neurofilament reduction, reactive gliosis. Whoever, concomitant rosemary intake and withdrawal of acrylamide modulate these effects. These findings proof that dietary rosemary can directly protect motor neuron against acrylamide toxicity in the mammalian developing spinal cord.
Keyword
MeSH Terms
Figure
Reference
-
1. Godoy M. Remember 'French fries cause cancer'? Here's the acrylamide update. Eating and health. The salt what is in your plate [Internet]. NPR;2013. cited 2016 Jan 1. Available from: http://www.npr.org/blogs/thesalt/2013/11/19/246188051/remember-death-by-french-fries-here-s-the-story.2. Carere A. Genotoxicity and carcinogenicity of acrylamide: a critical review. Ann Ist Super Sanita. 2006; 42:144–155.3. Giese JH. Acrylamide in foods. Food Technol. 2002; 56:71–75.4. Chen W, Feng L, Shen Y, Su H, Li Y, Zhuang J, Zhang L, Zheng X. Myricitrin inhibits acrylamide-mediated cytotoxicity in human Caco-2 cells by preventing oxidative stress. Biomed Res Int. 2013; 2013:724183.5. WHO/IPCS. 2006. Summary and conclusions of the sixty-fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) Rome, 8-17 February 2006 [Internet]. Geneva: World Health Organization;2006. cited 2016 Jan 1. Available from: http://www.who.int/ipcs/food/jecfa/summaries/en/i.6. Watson C, Kayalioglu G. The organization of the spinal cord. In : Watson C, Paxinos G, Kayalioglu G, editors. The Spinal Cord: A Christopher and Dana Reeve Foundation Text and Atlas. San Diego, CA: Academic Press;2010. p. 1–7.7. Mojska H, Gielecinska I, Szponar L, Oltarzewski M. Estimation of the dietary acrylamide exposure of the Polish population. Food Chem Toxicol. 2010; 48:2090–2096.8. Hogervorst JG, Baars BJ, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA. The carcinogenicity of dietary acrylamide intake: a comparative discussion of epidemiological and experimental animal research. Crit Rev Toxicol. 2010; 40:485–512.9. Motamedshariaty VS, Amel Farzad S, Nassiri-Asl M, Hosseinzadeh H. Effects of rutin on acrylamide-induced neurotoxicity. Daru. 2014; 22:27.10. El-Bakry AM, Abdul-Hamid M, Allam A. Prenatal and perinatal exposure of acrylamide disrupts the development of spinal cord in rats. World J Neurosci. 2013; 3:17–31.11. Hosseinzadeh H, Tabeshpur J, Mehri S. Effect of Saffron extract on acrylamide-induced toxicity: in vitro and in vivo assessment. Chin J Integr Med. (in press).12. Issabeagloo E, Kermanizadeh P, Taghizadieh M, Forughi R. Antimicrobial effects of rosemary (Rosmarinus officinalis L.) essential oils against Staphylococcus spp. Afr J Microbiol Res. 2012; 6:5039–5042.13. Inoue K, Takano H, Shiga A, Fujita Y, Makino H, Yanagisawa R, Kato Y, Yoshikawa T. Effects of volatile constituents of rosemary extract on lung inflammation induced by diesel exhaust particles. Basic Clin Pharmacol Toxicol. 2006; 99:52–57.14. Peter KV. Handbook of herbs and spices. Cambridge: Woodhead Publishing;2004. p. 250.15. Kosaka K, Yokoi T. Carnosic acid, a component of rosemary (Rosmarinus officinalis L.), promotes synthesis of nerve growth factor in T98G human glioblastoma cells. Biol Pharm Bull. 2003; 26:1620–1622.16. Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J, Kamins J, Okamoto S, Izumi M, Shirasawa T, Lipton SA. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008; 104:1116–1131.17. Chen X, Zhang Y, Zu Y, Yang L, Lu Q, Wang W. Antioxidant effects of rosemary extracts on sunflower oil compared with synthetic antioxidants. Int J Food Sci Technol. 2014; 49:385–391.18. El-Bakary NA, Mousa AM. Light and electron microscopic study on the effect of acrylamide on the cerebellar cortex of adult albino rat and the possible protective role of vitamin B6. Egypt J Histol. 2006; 29:179–192.19. Tyl RW, Marr MC, Myers CB, Ross WP, Friedman MA. Relationship between acrylamide reproductive and neurotoxicity in male rats. Reprod Toxicol. 2000; 14:147–157.20. Zohrabi M, Ashtiyani SC, Hajihashemi S, Hassanpoor A, Hosseini N. The study of 24 h post treatment effects of the aqueous extract of Rosmarinus officinalis after renal ischemia/reperfusion in rat. J Physiol Pathophysiol. 2012; 3:12–19.21. Sakr SA, Lamfon HA. Protective effect of rosemary (Rosmarinus officinalis) leaves extract on carbon tetrachloride-induced nephrotoxicity in albino rats. Life Sci J. 2012; 9:779–785.22. Dhungel S, Mukerjee B. Longitudinal study on the effect of chronic stresses on postnatal growth of the body and its constituent parts in male albino rat. J Anat Soc India. 2007; 56:18–24.23. LoPachin RM, Ross JF, Reid ML, Das S, Mansukhani S, Lehning EJ. Neurological evaluation of toxic axonopathies in rats: acrylamide and 2,5-hexanedione. Neurotoxicology. 2002; 23:95–110.24. Suvarna KS, Layton C, Bancroft JD. Bancroft's theory and practice of histological techniques. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier;2013. p. 172–214. p. 318–434.25. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974; 47:469–474.26. Kar M, Mishra D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976; 57:315–319.27. Liu Z, Martin LJ. Isolation of mature spinal motor neurons and single-cell analysis using the comet assay of early low-level DNA damage induced in vitro and in vivo. J Histochem Cytochem. 2001; 49:957–972.28. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988; 175:184–191.29. Wojewodzka M, Buraczewska I, Kruszewski M. A modified neutral comet assay: elimination of lysis at high temperature and validation of the assay with anti-single-stranded DNA antibody. Mutat Res. 2002; 518:9–20.30. De Muth JE. Basic statistics and pharmaceutical statistical applications. 3rd ed. Boca Raton, FL: CRC Press;2002. p. 61–95.31. El-Beltagi HS, Badawi MH. Comparison of antioxidant and antimicrobial properties for Ginkgo biloba and rosemary (Rosmarinus officinalis L.) from Egypt. Not Bot Horti Agrobot Cluj Napoca. 2013; 41:126–135.32. Annola K, Karttunen V, Keski-Rahkonen P, Myllynen P, Segerbäck D, Heinonen S, Vähäkangas K. Transplacental transfer of acrylamide and glycidamide are comparable to that of antipyrine in perfused human placenta. Toxicol Lett. 2008; 182:50–56.33. von Stedingk H, Rydberg P, Törnqvist M. A new modified Edman procedure for analysis of N-terminal valine adducts in hemoglobin by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2010; 878:2483–2490.34. Sorgel F, Weissenbacher R, Kinzig-Schippers M, Hofmann A, Illauer M, Skott A, Landersdorfer C. Acrylamide: increased concentrations in homemade food and first evidence of its variable absorption from food, variable metabolism and placental and breast milk transfer in humans. Chemotherapy. 2002; 48:267–274.35. Allam A, El-Gareeb A, Ajarem J, Abdul-Hamid M, El-Bakry A. Effect of acrylamide on the development of medulla oblongata in albino rat: biochemical and morphological studies. Afr J Pharm Pharmacol. 2013; 7:1320–1331.36. Mesembe OE, Ivang AE, Udo-Affah G, Igiri AO, Fischer VA, Akpaso M, Eluwa MA, Akpa OA. Morphometric study of the teratogenic effect of artesunate on the central nervous system of the Wistar rat foetus. Niger J Physiol Sci. 2005; 19:92–97.37. Wang H, Ge JY, Zhou ZQ, Wang ZC, Shi FX. Oral acrylamide affects the development and reproductive performance of male rats. Zhonghua Nan Ke Xue. 2007; 13:492–497.38. Ali AM. Pathological effect of acrylamide on rabbits [Ms thesis]. Markaz Rasheed: Department of Pathology, Faculty of Veterinary Medicine, Alexandria University;2008.39. Yao X, Yan L, Yao L, Guan W, Zeng F, Cao F, Zhang Y. Acrylamide exposure impairs blood-cerebrospinal fluid barrier function. Neural Regen Res. 2014; 9:555–560.40. Friedman MA, Tyl RW, Marr MC, Myers CB, Gerling FS, Ross WP. Effects of lactational administration of acrylamide on rat dams and offspring. Reprod Toxicol. 1999; 13:511–520.41. Neuromuscular Disease Center. Neuromuscular. Toxic neuropathesis: clinical and pathological features [Internet]. St. Louis, MO: Pestronk A, Washington University;c2015. cited 2016 Feb 1. Available from: http://neuromuscular.wustl.edu/nother/toxic.htm#acrylamide.42. Shaheed IB, Kawkab AA, Makhlouf MM. Toxicological and pathological studies on acrylamide neurotoxicity in albino rats. Egypt J Comp Pathol Clin Pathol. 2006; 19:63–82.43. El-Bakry AM, Abdul-Hamid M, Allam A. Prenatal and perinatal exposure of acrylamide disrupt the development of spinal cord in rats. World J Neurosci. 2013; 3:27902.44. Guo C, Li B, Xiao J. General survey of mechanisms of acrylamide neurotoxicity. Wei Sheng Yan Jiu. 2010; 39:282–285.45. Fleck F. Experts launch action on acrylamide in staple foods. BMJ. 2002; 325:120.46. Takahashi M, Shibutani M, Inoue K, Fujimoto H, Hirose M, Nishikawa A. Pathological assessment of the nervous and male reproductive systems of rat offspring exposed maternally to acrylamide during the gestation and lactation periods: a preliminary study. J Toxicol Sci. 2008; 33:11–24.47. Klaunig JE, Kamendulis LM. Mechanism of acrylamide induced rodent carcinogenesis. Adv Exp Med Biol. 2005; 561:49–62.48. Allam A, El-Ghareeb AA, Abdul-Hamid M, Baikry A, Sabri MI. Prenatal and perinatal acrylamide disrupts the development of cerebellum in rat: biochemical and morphological studies. Toxicol Ind Health. 2011; 27:291–306.49. Martyniuk CJ, Feswick A, Fang B, Koomen JM, Barber DS, Gavin T, Lopachin RM. Protein targets of acrylamide adduct formation in cultured rat dopaminergic cells. Toxicol Lett. 2013; 219:279–287.50. Yu S, Zhao X, Zhang T, Yu L, Li S, Cui N, Han X, Zhu Z, Xie K. Acrylamide-induced changes in the neurofilament protein of rat cerebrum fractions. Neurochem Res. 2005; 30:1079–1085.51. Wei X, Yan F, E M, Zhang C, Li G, Yang X, Zhang F, Wang S, Yu S. Neuroprotective effect of calpeptin on acrylamide-induced neuropathy in rats. Neurochem Res. 2015; 40:2325–2332.52. Fuhr U, Boettcher MI, Kinzig-Schippers M, Weyer A, Jetter A, Lazar A, Taubert D, Tomalik-Scharte D, Pournara P, Jakob V, Harlfinger S, Klaassen T, Berkessel A, Angerer J, Sörgel F, Schömig E. Toxicokinetics of acrylamide in humans after ingestion of a defined dose in a test meal to improve risk assessment for acrylamide carcinogenicity. Cancer Epidemiol Biomarkers Prev. 2006; 15:266–271.53. Moss M, Cook J, Wesnes K, Duckett P. Aromas of rosemary and lavender essential oils differentially affect cognition and mood in healthy adults. Int J Neurosci. 2003; 113:15–38.54. Erkan N, Ayranci G, Ayranci E. Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008; 110:76–82.55. Ito N, Yabe T, Gamo Y, Nagai T, Oikawa T, Yamada H, Hanawa T. Rosmarinic acid from Perillae Herba produces an antidepressant-like effect in mice through cell proliferation in the hippocampus. Biol Pharm Bull. 2008; 31:1376–1380.56. Ozarowski M, Mikolajczak PL, Bogacz A, Gryszczynska A, Kujawska M, Jodynis-Liebert J, Piasecka A, Napieczynska H, Szulc M, Kujawski R, Bartkowiak-Wieczorek J, Cichocka J, Bobkiewicz-Kozlowska T, Czerny B, Mrozikiewicz PM. Rosmarinus officinalis L. leaf extract improves memory impairment and affects acetylcholinesterase and butyrylcholinesterase activities in rat brain. Fitoterapia. 2013; 91:261–271.57. Fernandez S, Kurppa L, Hyvonen L. Content of acrylamide decreased in potato chips with addition of a proprietary flavonoid spice mix (Flavomere) in frying. Innov Food Technol. 2003; 18:24–26.58. International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risk to humans. Vol. 39. Some chemicals used in plastics and elastomers. Lyon: IARC Press;1985. p. 41–66.59. Christova-Bagdassarian VL, Ticshkova JA, Vrabcheva TM. Acrylamide in processed food. Bulg J Chem. 2012; 1:123–132.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of motor evoked potentials in the spinal cord injured rat
- Changes of motor evoked potentials and spinal cord evoked potentials following spinal cord injury in rats
- Differential gene expression pattern in brains of acrylamide-administered mice
- Effect of Cyclosporin A in a Rat Spinal Cord Injury Model
- Spinal Cord Atrophy and Early Motor Recovery following Transverse Myelitis in Pediatric Patients