Ann Rehabil Med.
2012 Jun;36(3):328-333. 10.5535/arm.2012.36.3.328.
Spinal Cord Atrophy and Early Motor Recovery following Transverse Myelitis in Pediatric Patients
- Affiliations
-
- 1Department of Rehabilitation Medicine, Seoul National University College of Medicine, Seoul 110-744, Korea. msbang@snu.ac.kr
- KMID: 2266737
- DOI: http://doi.org/10.5535/arm.2012.36.3.328
Abstract
OBJECTIVE
To compare the motor recovery following transverse myelitis in pediatric patients with and without spinal cord atrophy. METHOD: From January 1995 through December 2009, twenty children (8 boys and 12 girls with an onset at 5.7+/-3.8 years) that were diagnosed with transverse myelitis at a Children's Hospital in Korea, and undertaken an initial and follow-up spine magnetic resonance image (MRI) were included. Medical records and spine MRI scans were reviewed retrospectively. An initial MRI was taken 5.1+/-8.7 days after the onset. The interval between an initial and follow-up MRIs was 33.4+/-23.0 days. The motor recovery differences between subjects with and without spinal cord atrophy on follow-up MRIs were determined. Motor recovery was defined as the elevation of one or more grades of manual muscle tests of the Medical Research Council.
RESULTS
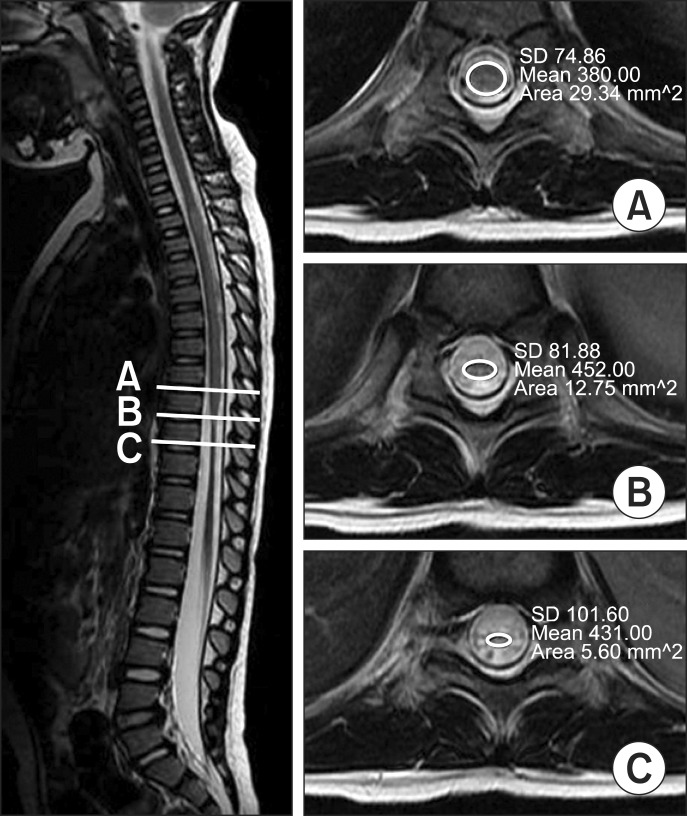
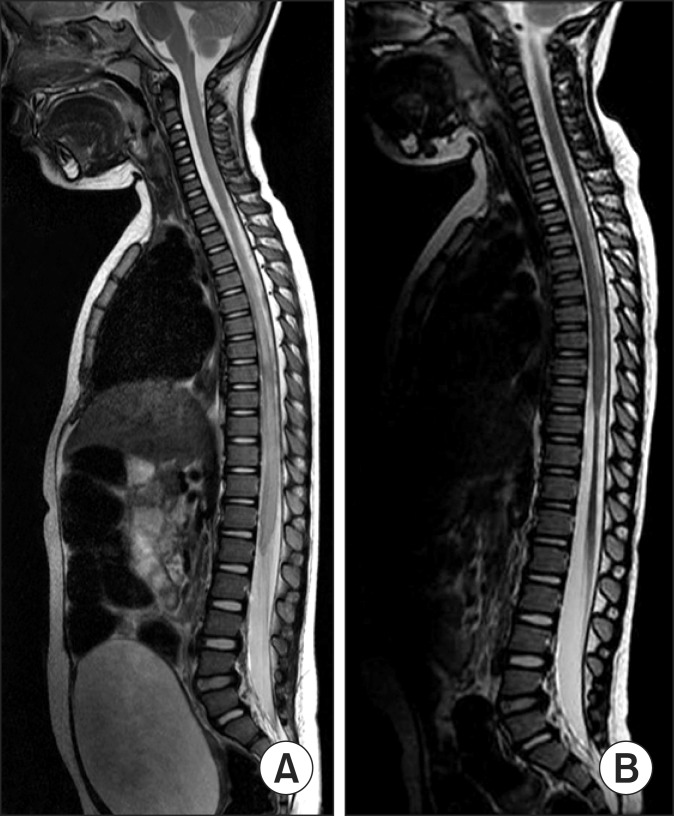
Eight patients had developed spinal cord atrophies and 12 patients had not. Of the 8 patients with spinal cord atrophy, 7 showed no motor improvement. Among the 12 patients without atrophy, 11 had motor improvement. Spinal cord atrophy on follow-up MRIs were related to the risk of no motor improvement (odds ratio=77.0, 95% confidence interval [4.114-1441.049], p-value=0.001).
CONCLUSION
Children with transverse myelitis who had developed spinal cord atrophy on follow-up MRIs had poor motor recovery than those who had not. The appearance of spinal cord atrophy on follow-up MRI could be an indicator of poor prognosis in pediatric transverse myelitis.
MeSH Terms
Figure
Reference
-
1. Dunne K, Hopkins IJ, Shield LK. Acute transverse myelopathy in childhood. Dev Med Child Neurol. 1986; 28:198–204. PMID: 3709989.
Article2. Berman M, Feldman S, Alter M, Zilber N, Kahana E. Acute transverse myelitis: incidence and etiologic considerations. Neurology. 1981; 31:966–971. PMID: 7196523.
Article3. Jeffery DR, Mandler RN, Davis LE. Transverse myelitis. Retrospective analysis of 33 cases, with differentiation of cases associated with multiple sclerosis and parainfectious events. Arch Neurol. 1993; 50:532–553. PMID: 8489410.4. Defresne P, Hollenberg H, Husson B, Tabarki B, Landrieu P, Huault G, Tardieu M, Sebire G. Acute transverse myelitis in children: clinical course and prognostic factors. J Child Neurol. 2003; 18:401–406. PMID: 12886975.
Article5. Kalra V, Sharma S, Sahu J, Sankhyan N, Chaudhry R, Dhawan B, Mridula B. Childhood acute transverse myelitis: clinical profile, outcome, and association with antiganglioside antibodies. J Child Neurol. 2009; 24:466–471. PMID: 19196873.
Article6. Bang MS, Kim SJ. Progression of spinal cord atrophy by traumatic or inflammatory myelopathy in the pediatric patients: case series. Spinal Cord. 2009; 47:822–825. PMID: 19172153.
Article7. Awerbuch G, Feinberg WM, Ferry P, Komar NN, Clements J. Demonstration of acute post-viral myelitis with magnetic resonance imaging. Pediatr Neurol. 1987; 3:367–369. PMID: 2853946.
Article8. Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002; 59:499–505. PMID: 12236201.9. Bruna J, Martinez-Yelamos S, Martinez-Yelamos A, Rubio F, Arbizu T. Idiopathic acute transverse myelitis: a clinical study and prognostic markers in 45 cases. Mult Scler. 2006; 12:169–173. PMID: 16629419.10. Knebusch M, Strassburg HM, Reiners K. Acute transverse myelitis in childhood: nine cases and review of the literature. Dev Med Child Neurol. 1998; 40:631–639. PMID: 9766742.
Article11. Irani DN, Kerr DA. 14-3-3 protein in the cerebrospinal fluid of patients with acute transverse myelitis. Lancet. 2000; 355:901. PMID: 10752712.
Article12. Braddom RL, Chan L, Harrast MA, Kowalske KJ, Matthews DJ, Ragnarsson KT, Stolp KA. Physical medicine and rehabilitation. 2011. 4th ed. Philadelphia: Elsevier Sounders;p. 47.13. Coulon O, Hickman SJ, Parker GJ, Barker GJ, Miller DH, Arridge SR. Quantification of spinal cord atrophy from magnetic resonance images via a B-spline active surface model. Magn Reson Med. 2002; 47:1176–1185. PMID: 12111964.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of diffuse spinal cord atrophy proven by MRI complicated by acute transverse myelitis
- A Case of Acute Transverse Myelitis Complicating Diffuse Spinal Cord Atrophy and Syrinx Formation
- Spinal cord stimulation for neuropathic pain following idiopathic transverse myelitis: A case report
- Motor Evoked Potentials in Transverse Myelitis
- MR Findings of Transverse Myelitis and Its Clinical Correlation