Korean J Hematol.
2007 Sep;42(3):258-263. 10.5045/kjh.2007.42.3.258.
Therapeutic Efficacy of a Single Dose of Anti-D Immunoglobulin 50 microgram/kg in Childhood Acute Immune Thrombocytopenic Purpura
- Affiliations
-
- 1Department of Pediatrics, Gil Medical Center, School of Medicine, Gachon University of Medicine and Science, Incheon, Korea. isjeon@gilhospital.com
- KMID: 2305201
- DOI: http://doi.org/10.5045/kjh.2007.42.3.258
Abstract
-
BACKGROUND: To investigate the efficacy and adverse effects of a single dose of anti-D immunoglobulin (50 microgram/kg) in Korean children with acute immune thrombocytopenic purpura (ITP).
METHODS
We serially evaluated the platelet count in 21 acute ITP patients that were treated with anti-D immunoglobulin (50 microgram/kg) to determine how many patients achieved platelet counts over the levels of 20 x 10(3)/mm3, 50 x 10(3)/mm3 and 100 x 10(3)/mm3 after the infusion of anti-D immunoglobulin. In addition, constitutional symptoms were monitored and changes in the hemoglobin levels were serially evaluated.
RESULTS
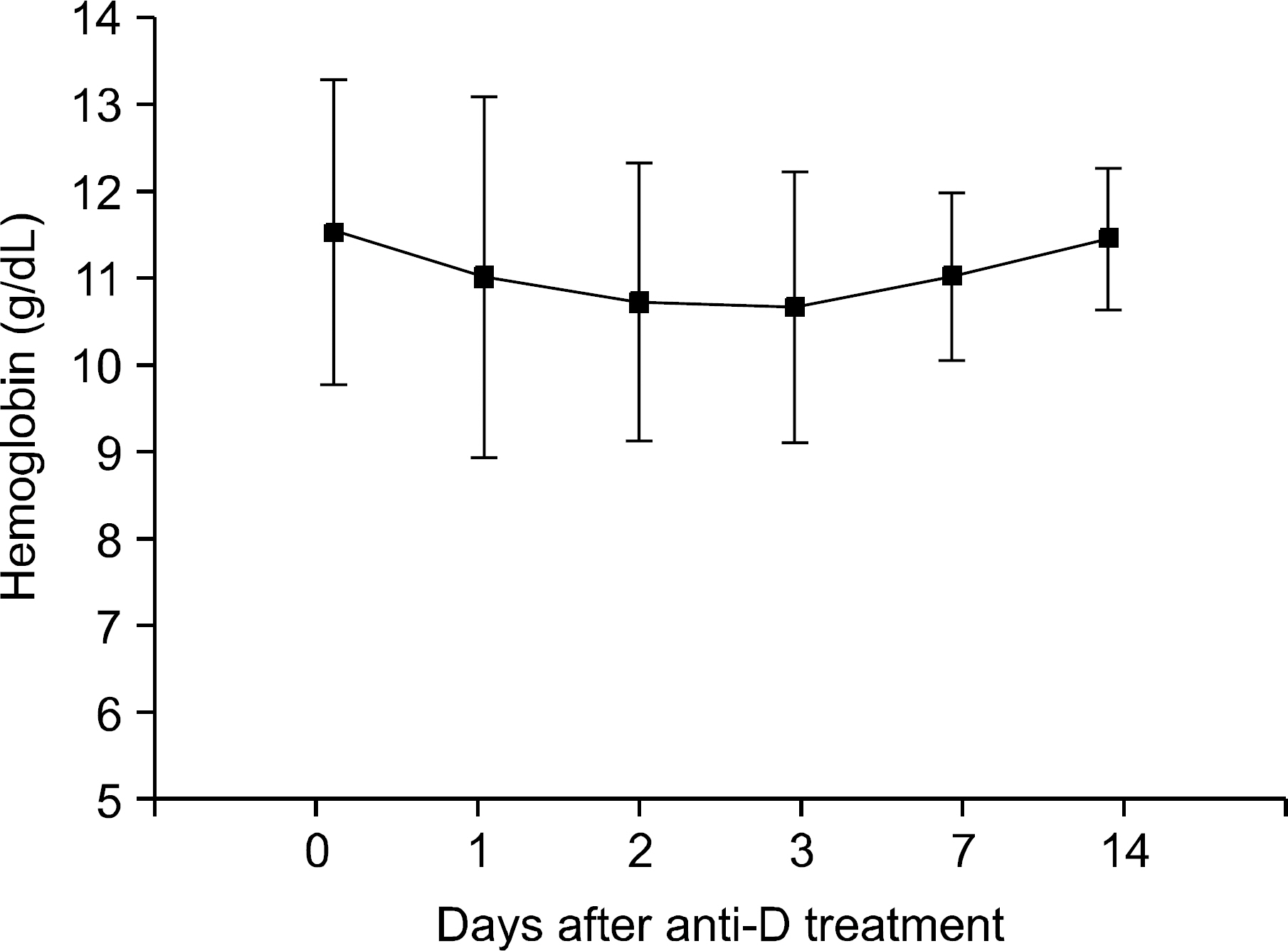
By three days after treatment, 90.5% of the patients had achieved a platelet count over 20 x 10(3)/mm3. At seven days after treatment, 66.7% of patients achieved a platelet count of 50 x 10(3)/mm3. In addition, at seven days after treatment 61.9% of patients achieved a platelet count of 100 x 10(3)/mm3. Constitutional adverse symptoms were observed 61.9% of patients, and the symptoms diminished spontaneously without any severe sequelaes. The decline of hemoglobin concentration after treatment recovered to the initial level after two weeks.
CONCLUSION
A single dose of anti-D immunoglobulin (50 microgram/kg)was effective in Korean children with acute ITP to raise the platelet count rapidly. The adverse effects of anti-D immunoglobulin, including hemolytic anemia, were not severe to prevent the use of the anti-D.
MeSH Terms
Figure
Reference
-
1). Kuhne T. Idiopathic thrombocytopenic purpura of childhood: a problem-oriented review of the management. Transfus Apher Sci. 2003. 28:243–8.2). Kuhne T., Imbach P., Bolton-Maggs PH., Berchtold W., Blanchette V., Buchanan GR. Newly diagnosed idiopathic thrombocytopenic purpura in childhood: an observational study. Lancet. 2001. 358:2122–5.3). George JN., Woolf SH., Raskob GE, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996. 88:3–40.4). Medeiros D., Buchanan GR. Major hemorrhage in children with idiopathic thrombocytopenic purpura: immediate response to therapy and long-term outcome. J Pediatr. 1998. 133:334–9.
Article5). Blanchette V., Imbach P., Andrew M, et al. Randomised trial of intravenous immunoglobulin G, intravenous anti-D, and oral prednisone in childhood acute immune thrombocytopenic purpura. Lancet. 1994. 344:703–7.
Article6). Tarantino MD., Madden RM., Fennewald DL., Patel CC., Bertolone SJ. Treatment of childhood acute immune thrombocytopenic purpura with anti-D immune globulin or pooled immune globulin. J Pediatr. 1999. 134:21–6.
Article7). Tarantino MD., Young G., Bertolone SJ, et al. Single dose of anti-D immune globulin at 75μg/kg is as effective as intravenous immune globulin at rapidly raising the platelet count in newly diagnosed immune thrombocytopenic purpura in children. J Pediatr. 2006. 148:489–94.
Article8). Park HY, Kang CG, Shin MY, Ahn KM, Sung KW, Koo HH, Treatment of childhood idiopathic thrombocytopenic purpura with anti-D (WinRhoⓇ). Korean J Pediatr Hematol-Oncol. 2000. 7:187–93.9). Hong F., Ruiz R., Price H., Griffiths A., Malinoski F., Woloski M. Safety profile of WinRho anti-D. Semin Hematol. 1998. 35:9–13.10). Gaines AR. Disseminated intravascular coagulation associated with acute hemoglobinemia or hemoglobinuria following Rh(0)(D) immune globulin intravenous administration for immune thrombocytopenic purpura. Blood. 2005. 106:1532–7.11). Christopher K., Horkan C., Barb IT., Arbelaez C., Hodgdon TA., Yodice PC. Rapid irreversible encephalopathy associated with anti-D immune globulin treatment for idiopathic thrombocytopenic purpura. Am J Hematol. 2004. 77:299–302.
Article12). Chun NS., Savani B., Seder RH., Taplin ME. Acute renal failure after intravenous anti-D immune globulin in an adult with immune thrombocytopenic purpura. Am J Hematol. 2003. 74:276–9.
Article13). Taube T., Schmid H., Reinhard H., von Stackelberg A., Overberg US. Effect of a single dose of rituximab in chronic immune thrombocytopenic purpura in childhood. Haematologica. 2005. 90:281–3.14). Wang J., Wiley JM., Luddy R., Greenberg J., Feu-erstein MA., Bussel JB. Chronic immune thrombocytopenic purpura in children: assessment of rituximab treatment. J Pediatr. 2005. 146:217–21.
Article15). Jenkins JM., Williams D., Deng Y, et al. Phase 1 clinical study of eltrombopag, an oral, nonpeptide thrombopoietin receptor agonist. Blood. 2007. 109:4739–41.
Article16). Bussel JB., Kuter DJ., George JN, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006. 355:1672–81.
Article17). Wang B., Nichol JL., Sullivan JT. Pharmacodynamics and pharmacokinetics of AMG 531, a novel thrombopoietin receptor ligand. Clin Pharmacol Ther. 2004. 76:628–38.
Article18). Ware RE., Zimmerman SA. Anti-D: mechanisms of action. Semin Hematol. 1998. 35:14–22.19). Imbach P., Barandun S., d'Apuzzo V, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981. 1:1228–31.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Severe Hemolytic Anemia following Anti-D Immunoglobulin Administration for Acute Immune Thrombocytopenic Purpura in Two-month-old Infants
- Treatment of Immue Thrombocytopenic Purpura in Childhood
- Efficacy of Very Low-dose (200 mg/kg/d) with Short-term Intravenous Immunoglobulin G Therapy according to Individual Response of Acute Immune Thrombocytopenic Purpura in Childhood
- Low-dose and Short-term Therapy of Intravenous Immunoglobulin G for Childhood Acute Immune Thrombocytopenic Purpura (1)
- An Analysis of Neutropenia after the Administration of High-dose Intravenous Immunoglobulin or Anti-D Immunoglobulin on Acute Immune Thrombocytopenic Purpura Children: Age based Analysis